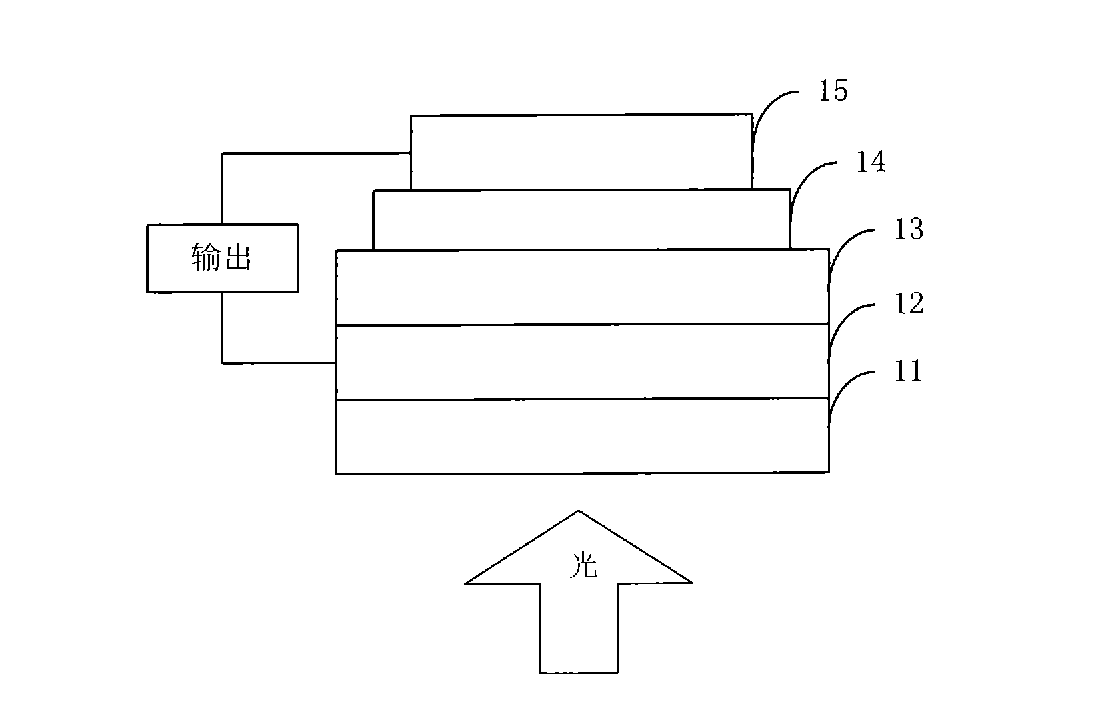
Metalloporphyrin-thienopyrazine organic semiconductor material, preparation method thereof and application thereof
A technology of organic semiconductors and metalloporphyrins, applied in semiconductor/solid-state device manufacturing, semiconductor devices, luminescent materials, etc., can solve the problems of limiting the application range of organic semiconductor materials, lack of literature and patent reports, etc., and achieve good thermal stability and Effects of environmental stability, improvement of carrier mobility, and expansion of application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of metalloporphyrin-thienopyrazine organic semiconductor material designed by the present invention, the steps are as follows:
[0045] Step S1: Dissolving dipyrromethane (A), the first silafluorene derivative (B) and the second silafluorene derivative (C) in a molar ratio i:j:k in the first organic compound containing the oxidizing agent and the first catalyst In the solvent, add an oxidizing agent and a first catalyst to the first organic solvent, and react at 20-100° C. for 1-24 hours to obtain a silicon fluorene porphyrin derivative (D), wherein, i:j:k= 1: 1~100: 1~100, and i=j+k, i≥j>0; the first catalyst adopts organic acid, as propionic acid, trifluoroacetic acid; Oxidant can adopt dichlorodicyanobenzoquinone (DDQ ), the first organic solvent can be one or both in chloroform or dichloromethane; Reaction formula is as follows:
[0046]
[0047] Step S2: Dissolve the silicon fluorene porphyrin derivative (D) obtained in step S1 and the br...
Embodiment 1
[0066] This embodiment discloses a silicon fluorene zinc porphyrin-thienopyrazine organic semiconductor material with the following structure
[0067]
[0068] In the above formula, n=10;
[0069] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0070] 1. Synthesis of 5,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) base thieno[3,4-b]pyrazine
[0071]
[0072] Under the protection of argon, add p-5,7-dibromothieno[3,4-b]pyrazine (8.8g, 0.03mol) into the three-necked flask, add 200ml of tetrahydrofuran solvent, and at -78°C Slowly inject n-butyllithium (25.2mL, 2.5M, 0.06mol) with a syringe, continue to stir the reaction for 2h, inject 2-isopropoxy-4,4,5,5- Tetramethyl-1,3,2-dioxaborolane (13 mL, 0.06 mol), stirred overnight at room temperature. Saturated aqueous sodium chloride (30ml) was added to terminate the reaction, extracted with chloroform, dried over anhydrous sodium sulfate, and filtered, the filtrate was collected...
Embodiment 2
[0090] This embodiment discloses a silicon fluorene iron porphyrin-thienopyrazine organic semiconductor material with the following structure
[0091]
[0092] In the above formula, n=28;
[0093] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0094] 1. Synthesis of 5,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) base thieno[3,4-b]pyrazine
[0095] Its preparation sees embodiment 1 for details.
[0096] 2. Synthesis of 5-(9'-methyl-9'-hexadecyl)silafluorene-15-(9'-docosyl)silafluorene porphyrin
[0097]
[0098] Set up an anhydrous and oxygen-free device, weigh the intermediates 2-aldehyde-9-methyl-9-hexadecylsilafluorene (0.45g, 1mmol), 2-aldehyde-9-docosylsilafluorene ( 0.66g, 1mmol), dipyrromethane (0.30g, 2mmol), dissolved in 250ml of dichloromethane, helium gas for 30min, add 2ml of trifluoroacetic acid into the syringe, stir at 100°C for 1h, then add dichlorodicyano Benzoquinone (DDQ) (1.82g, 8mmol), continued to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com