Preparation method for beta-lactamase inhibitory polypeptide and expression vector used therein
A technology for inhibiting polypeptides and lactamases, which is applied in the fields of expression vectors, preparation of beta-lactamase inhibitory polypeptides, and fusion proteins, can solve the problems of high cost and difficulty in purification, and achieves low price, difficulty in purification and cost reduction. The ideal effect of enzyme digestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Construction and protein expression of embodiment 1 expression vector
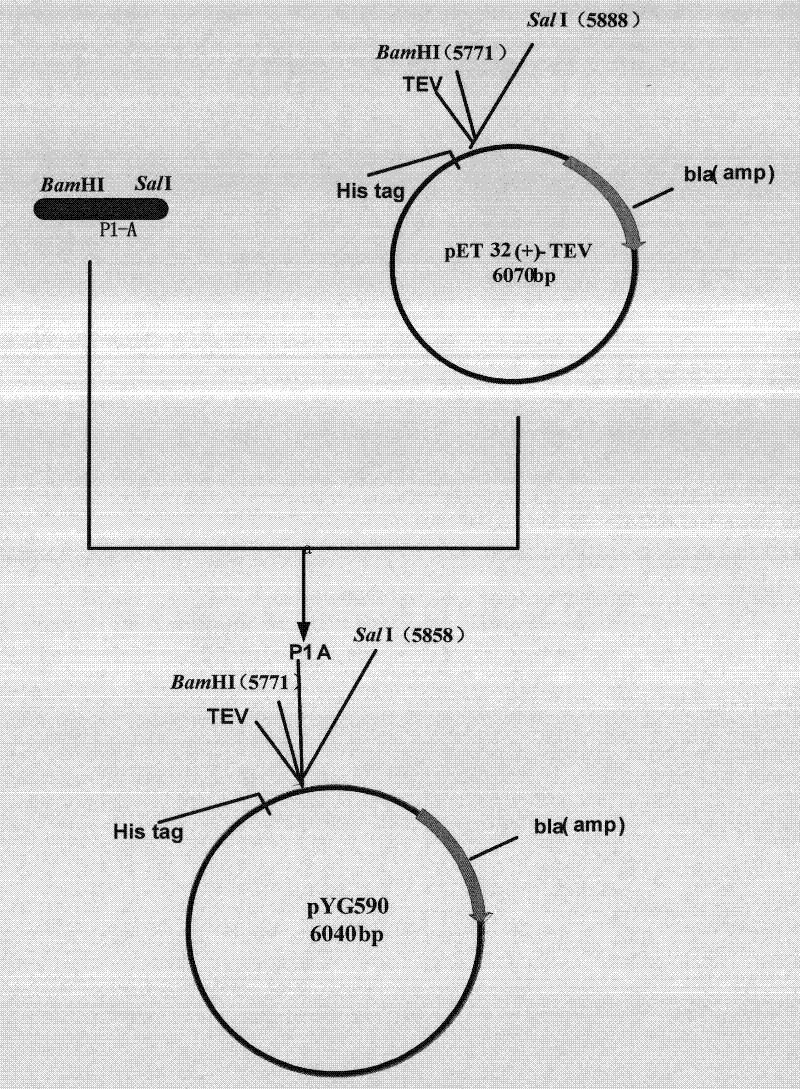
[0051] 1) Construction of pYG590
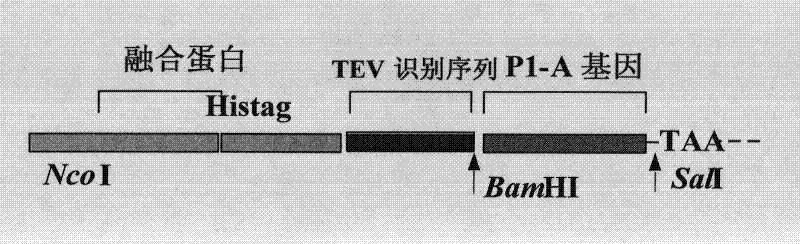
[0052] The TEV enzyme specifically recognizes the amino acid sequence of Glu-Asn-Lys-Tyr-Phe-Gln-Gly and cuts between Gln-Gly. The idea of constructing the new vector is to combine the coding sequence of the recognition site of the TEV enzyme with the polypeptide P1 -A gene is connected and then cloned into the expression plasmid, the schematic diagram is shown in figure 1 .
[0053] Primers Primer5 and Primer6 with restriction sites for endonucleases BamHI and SalI were designed respectively (see Table 1). The polypeptide P1-A gene was amplified using pYG567 (see Chinese patent application 200910054340.1) as a template. The amplified product was double-digested with BamHI and SalI, and then combined with the pET32a-TEV plasmid (pET32a-TEV plasmid is the TEV recognition site cloned by the inventor of 7 amino acid sequences in pET32a, which was prepared The me...
Embodiment 2
[0080] The separation and purification of embodiment 2 small peptides
[0081] 1) TEV enzyme digests P1-A-TEV fusion protein
[0082] The purified P1-A-TEV fusion protein was appropriately concentrated to more than 1.0 mg / ml with a 10KDa ultrafiltration tube ultrafiltration (Amicon Ultra10K device, MILLIPORE Company), and the newly purified TEV enzyme in Example 1 was used according to the following table 2 The system shown in the enzyme cleavage reaction, 34 ° C water bath reaction 1-1.5h, the cleavage efficiency can exceed 50%.
[0083] Table 2. TEV enzyme digestion reaction system
[0084] 10× Enzyme Digestion Buffer
200μl
fusion protein
1.2-1.8mg
TEV enzyme
0.6-0.9mg
total capacity
2000μl
[0085] Among them, the 10× digestion buffer (1ml) is as follows:
[0086]
[0087] Using Tricine-SDS-PAGE electrophoresis detection, it can be seen that the P 1-A-TEV fusion protein is cut, and a small band with a size of about...
Embodiment 3P1
[0094] Example 3 In vivo inhibition experiment of P1-A-TEV fusion protein and polypeptide P1-A on drug-resistant bacteria
[0095] In order to detect whether the P1-A-TEV fusion protein obtained by the expression of the newly constructed vector pYG590 and the polypeptide P1-A have β-lactamase inhibitory activity, Klebsiella pneumoniae 10032 (ie ATCC700603) was used as the test bacteria for in vivo inhibition In the test, the β-lactamase inhibitor potassium clavulanate (purchased from Shanghai Xibao Biotechnology Co., Ltd., with a purity of 99.9%, meeting the standards of the United States Pharmacopoeia) was used as a control.
[0096] Firstly, the P1-A-TEV fusion protein purified from the broken supernatant, the P1-A-TEV fusion protein obtained by renaturation purification, the P1-A obtained by ultrafiltration and clavula were investigated as described in Example 2. Effect of potassium phosphate on the growth of Klebsiella pneumoniae. The P1-A-TEV fusion protein or polypeptid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com