Method for differentiating and culturing mononuclear cells into mature dendritic cells
A technology of dendritic cells and monocytes, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problems of expensive transwell, expensive cytokines, long cell induction cycle, etc., to overcome Cultivate complexity and instability, optimize the culture system, and the effect of mass culture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Construction of Ea.hy926 cells secreting GM-CSF and IL-4, and TNF-α
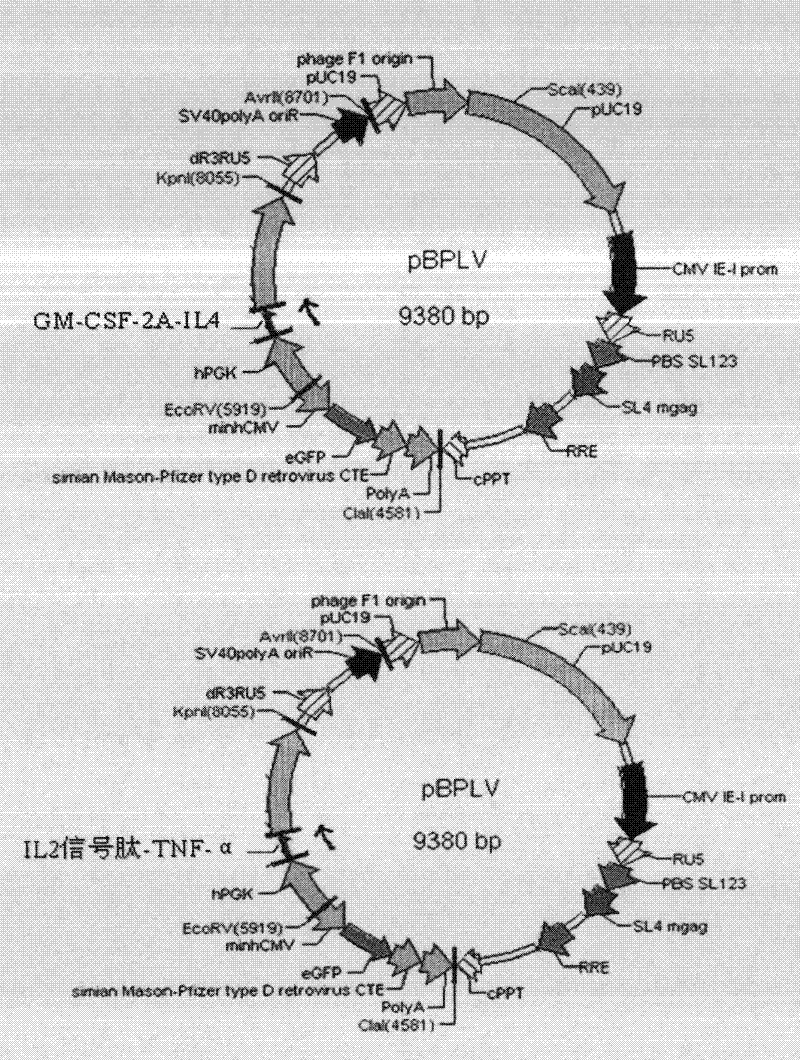
[0031] 1. Establishment of lentiviral multi-gene co-expression vector
[0032] 1) Amplify GM-CSF-2A and 2A-IL4 sequences respectively.
[0033] 2A sequence forward primer: 5'-3'
[0034] GAGGGCAGAGGAAGTCTTCTAACATGCGGTGACGTGGAGGAGAATCCCGGCCCT
[0035] 2A sequence reverse primer: 5'-3'
[0036] AGGGCCGGGATTCTCCTCCACGTCACCGCATGTTAGAAGACTTCCTCTGCC CTC
[0037] 2) Amplify the fusion gene GM-CSF-2A-IL4.
[0038] 3) Amplify the IL2 signal peptide-sTNF-α.
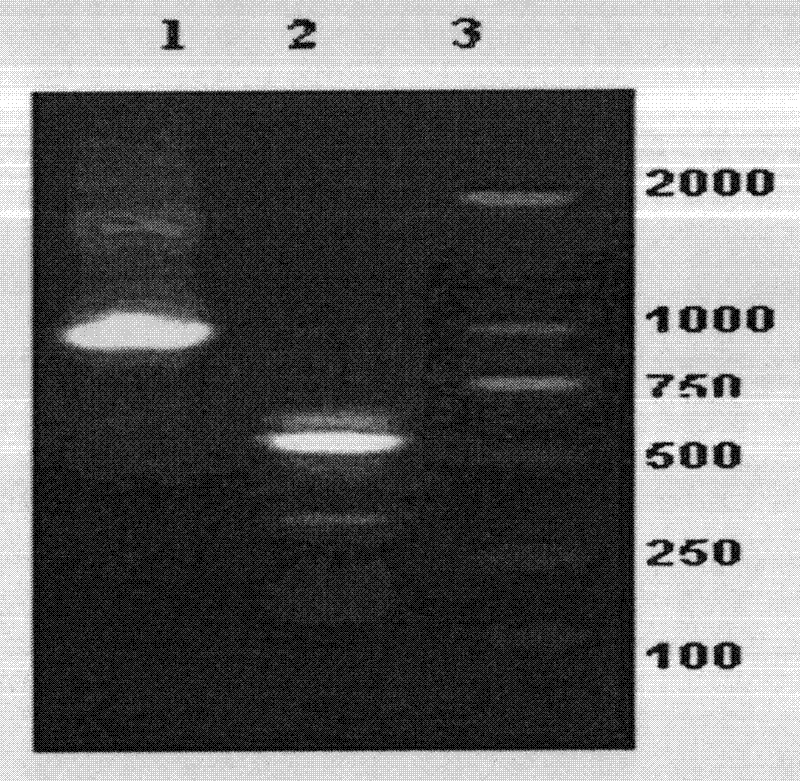
[0039] 2) and 3) PCR amplification results see figure 1 ,exist figure 1 Among them, each lane is (1). GM-CSF-2A-IL4 overlapping PCR results; (2) IL2 signal peptide-sTNF-α; (3) molecular weight marker DL.marker2000. By polymerase chain reaction (PCR), 948bp of GM-CSF and IL4 co-expressed genes, and about 530bp of the target gene IL2 signal peptide-sTNF-α were obtained. The sequencing results were consistent with the sequence published by ...
Embodiment 2
[0064] Example 2 Preparation of feeder layer cells
[0065] 1) Infect Ea.hy926 cells with the above-mentioned lentivirus to obtain IL4, GM-CSF-Ea.hy926 feeder cells and TNF-α-Ea.hy926 feeder cells;
[0066] The infection steps are the same as those in Example 1 for infecting EA.hy926 cells with lentivirus;
[0067] 2) Using fluorescent cell sorting technology, sort out GFP strong expressing strains and weak expressing strains according to the intensity of GFP fluorescence;
[0068] 3) IL4, GM-CSF-Ea.hy926 and TNF-α-Ea.hy926 cells were irradiated with gamma rays at a dose of 40Gy to inhibit their proliferation, and the irradiated cells were replaced with medium, incubated in a cell culture box for 2-3h, PBS solution Wash and prepare feeder cells.
Embodiment 3
[0069] Example 3 PBMC and feeder layer cell co-culture
[0070] 1) Preparation of normal human peripheral blood PBMC (operated according to the instructions of the lymphocyte separation medium kit, human lymphocyte separation medium Product Number: LTS 1077, purchased from Haoyang Biotechnology Company, Institute of Bioengineering, Chinese Academy of Medical Sciences);
[0071] 2) Sorting peripheral blood CD 14 by immunomagnetic bead method + Monocytes (use Monocyte Isolation Kit II Order no.130-091-153 from Miltenyi, Germany, and isolate according to the instructions);
[0072] 3) After the GM-CSF and IL4-Ea.hy926 feeder cells adhere to the wall, inoculate PBMC cells, collect the suspended cells for 48 hours and inoculate them on the TNF-α-Ea.hy926 feeder layer cells, and collect the suspended cells after 24 hours
[0073] The cells are mature DCs.
[0074] Figure 7 To observe the morphology of DC cultured in vitro with an inverted microscope, by Figure 7 It can be seen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com