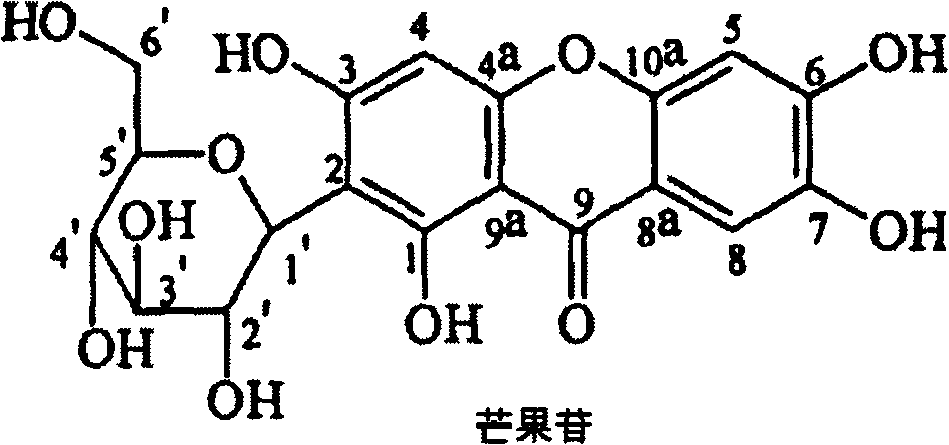
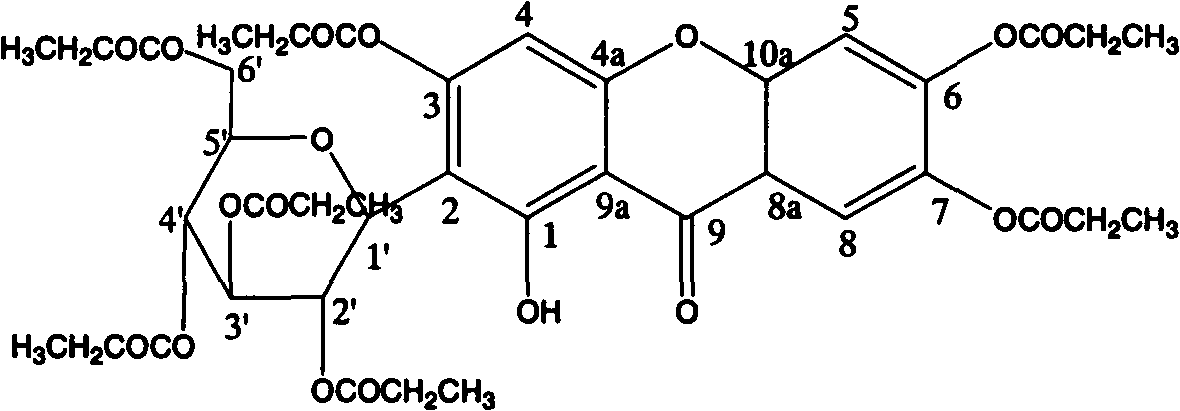
Mangiferin hepta-propyl-esterified derivative
A compound, the technology of propionic anhydride, applied in the field of mangiferin heptapropyl esterification derivatives, can solve the problems of poor gastrointestinal permeability of mangiferin, poor permeability of mangiferin, poor absorption effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Preparation of mangiferin heptapropyl esterified derivatives
[0013] Operate in a water bath, add 3ml of 98% H 2 SO 4 , then add 10g of mangiferin after dripping; place the solution at 60°C to keep warm, react for 36 hours, stir from time to time to dissolve all mangiferin; after the reaction is complete, pour the reaction solution directly into water under constant stirring ; The insoluble matter was filtered to obtain the reaction product; the reaction product was mixed with silica gel, put on the top of the silica gel dry column, and was first eluted with chloroform, then eluted with chloroform: methanol=25: 1, collected fractions, evaporated to dryness, Obtain crude crystals; recrystallize the crude crystals with methanol 2-3 times to obtain mangiferin heptapropyl esterified derivatives.
Embodiment 2
[0014] Example 2: Preparation of mangiferin heptapropyl esterified derivatives
[0015] Operate in a water bath, add 10ml of 36% HCl dropwise to 250ml of propionic anhydride under constant stirring, and then add 10g of mangiferin after dropping; keep the solution at 70°C for 18 hours of reaction, stirring from time to time Dissolve the mangiferin completely; after the reaction is completed, pour the reaction solution directly into water under constant stirring; filter the insoluble matter to obtain the reaction product; mix the reaction product with silica gel, put it on the top of the silica gel dry column, and first use chloroform Elution, followed by elution with chloroform:methanol=25:1, collected fractions, evaporated to dryness, to obtain crude crystals; the crude crystals were recrystallized 2-3 times with methanol to obtain mangiferin heptapropyl esterified derivatives.
Embodiment 3
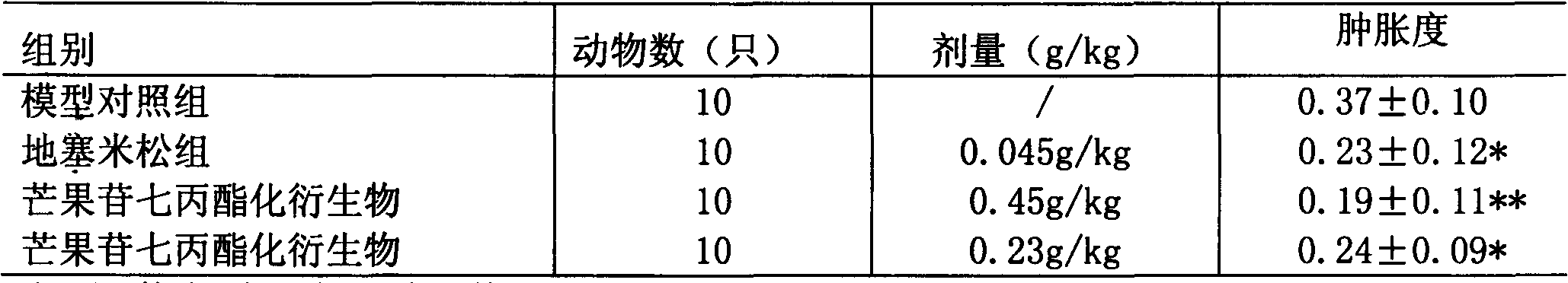
[0016] Embodiment 3: anti-inflammatory pharmacological experiment and result
[0017] Mouse auricle swelling test 40 mice were randomly divided into 4 groups. Set up normal control group, aspirin group (0.2g / kg), mangiferin heptapropyl ester derivative group (0.45g / kg, 0.25g / kg), intragastric administration once a day, normal control group The group was given an equal volume of distilled water for 7 consecutive days. 0.5 h after the last administration, 20 μl of xylene was dripped on the right ear of the mouse with a micro-syringe, and the mouse was killed by pulling the cervical spine 15 min later. Immediately use a hole punch with a caliber of 6 mm to punch holes along the same part of the left and right auricles of the mice, and then weigh them with an electronic balance of 1 / 10,000, and take the difference in weight (mg) between the two ears as the index of swelling degree , swelling degree=(right ear weight-left ear weight) / right ear weight, the results are shown in Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com