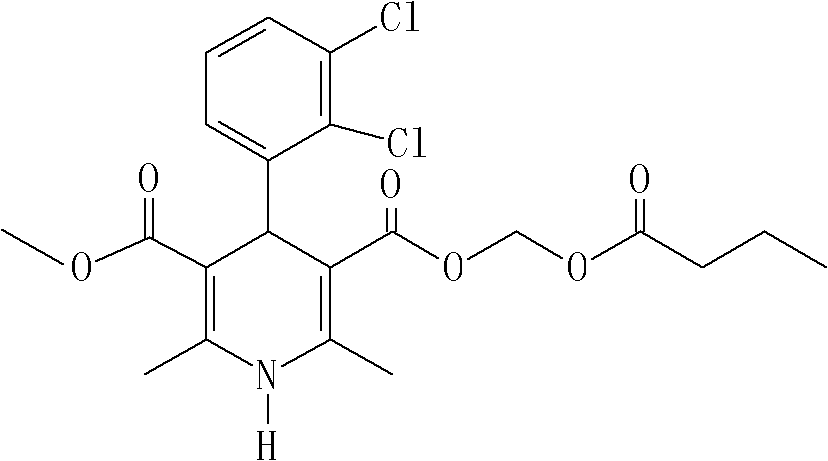
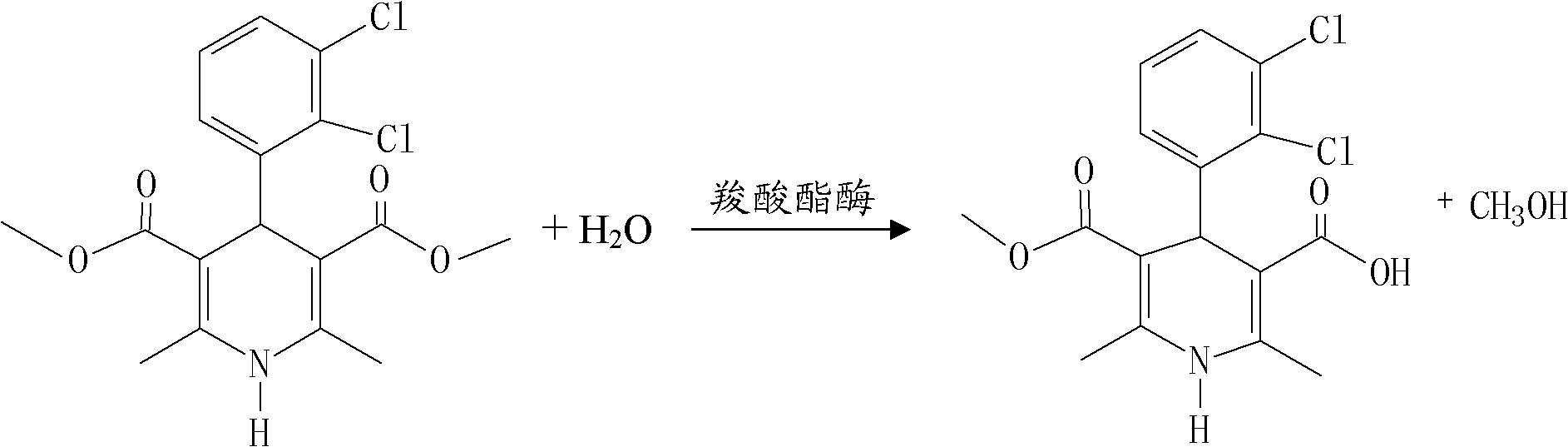
Method for preparing clevidipine butyrate
A technology of ammonium acetate and dichlorophenyl, applied in the field of preparation of clevidipine butyrate, can solve the problems of slow reaction speed, low total yield, low melting point, etc. The effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 2,3-dichlorobenzaldehyde (17.5g, 0.1mol), ammonium acetate (11.6g, 0.15mol), methyl acetoacetate (17.3g, 0.15mol) to the ultrasonic reactor, set the reaction power to 100W, and react 5h. Release the reaction liquid from the ultrasonic reactor, cool to below 10°C, stir for 2 hours to precipitate crystals, filter, and wash the crystals with water three times. Then, the obtained crystals were completely dissolved in 100ml of absolute ethanol at 50-60°C, placed at room temperature for 1 hour, and then placed in a refrigerator at 0-5°C for 8 hours. Filter, and dry the obtained crystals in an oven at 70-80° C. for 6 hours to obtain 33.2 g of light yellow sand-like crystals (Intermediate I) (content: 98.4%, yield: 89.5%).
[0025] Melting point: 187.0~188.5℃
[0026] IR (KBr, cm -1 ): 3336, 2948, 1704, 1680, 1651, 1620, 1502, 1286, 779, 732.
[0027] Utilize HPLC to carry out assay, detection condition is as follows:
[0028] Column: C 8 (250mm×4.6mm, 5um) (Welch Mat...
Embodiment 2
[0034] Add 2,3-dichlorobenzaldehyde (17.5g, 0.1mol), ammonium acetate (11.6g, 0.15mol), methyl acetoacetate (17.3g, 0.15mol) to the ultrasonic reactor, set the reaction power to 500W, and react 1h. Release the reaction liquid from the ultrasonic reactor, cool to below 10°C, stir for 2 hours to precipitate crystals, filter, and wash the crystals with water three times. Then, the obtained crystals were completely dissolved in 100ml of absolute ethanol at 50-60°C, placed at room temperature for 1 hour, and then placed in a refrigerator at 0-5°C for 8 hours. Filter and dry the obtained crystals in an oven at 70-80°C for 6 hours to obtain 30.2 g of light yellow sandy crystals (Intermediate I) (content: 98.9%, yield: 81.4%).
[0035] Melting point: 186.9~187.9℃
[0036] IR (KBr, cm -1 ): 3334, 2949, 1705, 1681, 1651, 1620, 1502, 1286, 779, 733.
[0037] Utilize HPLC to carry out content determination, and determination condition is identical with embodiment 1.
Embodiment 3
[0039] Add 2,3-dichlorobenzaldehyde (17.5g, 0.1mol), ammonium acetate (23.2g, 0.30mol), methyl acetoacetate (17.3g, 0.15mol) to the ultrasonic reactor, set the reaction power to 250W, and react 2h. Release the reaction liquid from the ultrasonic reactor, cool to below 10°C, stir for 2 hours to precipitate crystals, filter, and wash the crystals with water three times. Then, the obtained crystals were completely dissolved in 100ml of absolute ethanol at 50-60°C, placed at room temperature for 1 hour, and then placed in a refrigerator at 0-5°C for 8 hours. Filter, and dry the obtained crystals in an oven at 70-80° C. for 6 hours to obtain 34.8 g of light yellow sand-like crystals (Intermediate I) (content: 98.6%, yield: 93.8%).
[0040] Melting point: 187.0~188.2℃
[0041] IR (KBr, cm -1 ): 3336, 2949, 1704, 1680, 1651, 1620, 1502, 1286, 779, 732.
[0042] The HPLC detection conditions were the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com