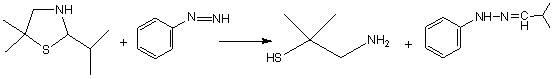
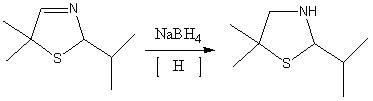Synthetic method of dimethyl cysteamine hydrochloride
A technology of dimethylcysteamine hydrochloride and its synthesis method, which is applied in the field of synthesis of dimethylcysteamine hydrochloride, can solve problems such as long reaction cycle and unsatisfactory product quality, and achieve low component cost , Low requirements for reaction equipment and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
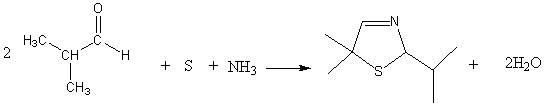
[0033] 1. Synthesis of 5,5-dimethyl-2-isopropylthiazoline reaction
[0034] In the autoclave, 144g of isobutyraldehyde, 81g of triethylamine, and 32g of elemental sulfur were mixed uniformly.
[0035] Discharge the mixed gas to -0.05MPa by vacuum decompression, open the circulating cooling water, keep the temperature below 40°C, feed ammonia gas to pressurize to 0.03MPa, stop feeding ammonia gas, open the vent valve, and discharge excess ammonia gas to Normal pressure, repeated charging and discharging 5 times, finally reaching normal pressure after deflation.
[0036] Keep circulating cooling water and continue to fill with 34gNH 3 , close the intake valve, stop cooling, start heating to 110°C, and keep for 4h. Stop heating, turn on circulating cooling water, cool down to 20°C, and repeat the above process.
[0037] Stop the reaction, open the inlet valve, release the product under pressure, the solution is light yellow or brown. After filtration, let it stand for liqui...
Embodiment 2
[0046] 1. Synthesis of 5, 5-dimethyl-2-isopropylthiazoline reaction
[0047] In the autoclave, 144g of isobutyraldehyde, 81g of triethylamine, and 32g of elemental sulfur were mixed evenly.
[0048] Use vacuum to depressurize to -0.05MPa to discharge the mixed gas, open the circulating cooling water, keep the temperature below 40°C, feed ammonia gas and pressurize to 0.03MPa, stop feeding ammonia gas, open the vent valve, and discharge excess ammonia gas to Normal pressure, repeated charging and discharging 5 times.
[0049] Keep circulating cooling water, continue to charge 68 g NH 3 ; Close the intake valve, stop cooling, start heating at a rate of 0.5°C / min to 110°C, and keep for 4h.
[0050] Stop the reaction, open the vent valve, and discharge the remaining waste gas of the reaction; open the intake valve, fill with nitrogen and pressurize to discharge the product, the solution is light yellow or brown. After filtering, let stand to separate liquid to remove water ph...
Embodiment 3
[0059] 1. Synthesis of 5, 5-dimethyl-2-isopropylthiazoline reaction
[0060] In the autoclave, 144g of isobutyraldehyde, 81g of triethylamine, and 32g of elemental sulfur were mixed evenly.
[0061] Use vacuum to depressurize to -0.05MPa to discharge the mixed gas, open the circulating cooling water, keep the temperature below 40°C, feed ammonia gas and pressurize to 0.03MPa, stop feeding ammonia gas, open the vent valve, and discharge excess ammonia gas to Normal pressure, repeated charging and discharging 5 times.
[0062] Keep circulating cooling water and continue to fill with 51 g NH 3 ; Close the intake valve, stop cooling, start heating at a rate of 0.5°C / min to 100°C, and keep for 8 hours.
[0063] Stop the reaction, open the vent valve, and discharge the remaining waste gas of the reaction; open the intake valve, fill with nitrogen and pressurize to discharge the product, the solution is light yellow or brown. After filtering, let stand to separate liquid to remo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com