Alpha,beta-diamino acid derivative and synthetic method and application thereof
A technology of diamino acid and synthetic method, applied in the field of alpha, can solve the problems of unfriendly environment, high cost, poor universality of substrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
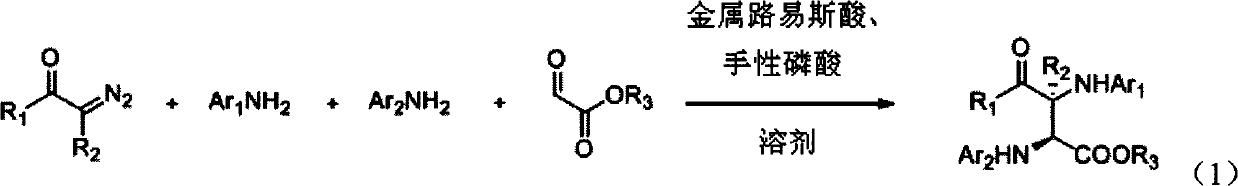
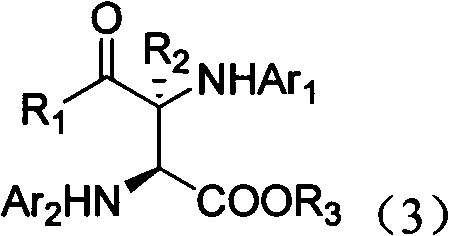
[0065] Ethyl glyoxylate (0.20mmol, 1.0eq), PhNH 2 (0.44mmol, 2.2eq), Rh 2 (OAc) 4 (0.004mmol), chiral phosphoric acid (0.01mmol) and Molecular sieves (100mg) were dissolved in toluene (1.0ml) at room temperature and stirred for 10min; then, diazoacetophenone (0.24mmol, 1.2eq) dissolved in toluene (1.0ml) was added dropwise within 1 hour Into the reaction system, after the dropwise addition, stir for 20 minutes. The reaction product was subjected to column chromatography (ethyl acetate:petroleum ether=1:15~1:10) to obtain a pure product. The structure of the product is shown in formula 3-1. The yield was 95%, the dr value was greater than 90:10, and the ee value was 95%.
[0066] Wherein, the chiral phosphoric acid substituent R is triphenylsilyl.
[0067] 1 H-NMR (500MHz, CDCl 3 ): 8.00(d, J=6.5Hz, 2H), 7.63(m, 1H), 7.52(d, J=6.5Hz, 2H), 7.16-7.21(m, 4H), 6.78-6.81(m, 4H) , 6.61-6.63(m, 2H), 5.54-5.56(m, 1H), 4.79(d, 2H), 4.62-4.68(m, 2H), 4.04(m, 2H), 1....
Embodiment 2
[0069]
[0070] Ethyl glyoxylate (0.2mmol, 1.0eq), P-ClPhNH 2 (0.44mmol, 2.2eq), Rh 2 (OAc) 4 (0.004mmol), chiral phosphoric acid (0.01mmol) and Molecular sieves (200mg) were dissolved in toluene (1.0ml) at room temperature and stirred for 10min; then, diazoacetophenone (0.24mmol, 1.2eq) dissolved in toluene (3.0ml) was added dropwise within 1 hour Into the reaction system, after the dropwise addition, stir for 20 minutes. The reaction product was subjected to column chromatography (ethyl acetate:petroleum ether=1:15~1:10) to obtain a pure product. The structure of the product is shown in formula 3-2. The yield was 90%, the dr value was greater than 90:10, and the ee value was 94%.
[0071] Wherein, the chiral phosphoric acid substituent R is triphenylsilyl.
[0072] 1 H-NMR (400MHz, CDCl 3 ): 7.95-7.97(m, 2H), 7.62-7.66(m, 1H), 7.49-7.52(m, 2H), 7.11-7.17(m, 2H), 7.08-7.09(m, 2H), 6.70-6.73 (m, 2H), 6.51-6.54(m, 2H), 5.45-5.49(m, 1H), 4.76(d, J=10.2Hz, 1H), 4.64(...
Embodiment 3
[0074]
[0075] Ethyl glyoxylate (0.2mmol, 1.0eq), p-BrPhNH 2 (0.44mmol, 2.2eq), Rh 2 (OAc) 4 (0.004mmol), chiral phosphoric acid (0.01mmol) and Molecular sieves (150mg) were dissolved in toluene (1.0ml) at room temperature and stirred for 10min; then, diazoacetophenone (0.24mmol, 1.2eq) dissolved in toluene (2.0ml) was added dropwise within 1 hour Into the reaction system, after the dropwise addition, stir for 20 minutes. The reaction product was subjected to column chromatography (ethyl acetate:petroleum ether=1:15~1:10) to obtain a pure product. The structure of the product is shown in formula 3-3. The yield was 89%, the dr value was greater than 90:10, and the ee value was 92%.
[0076] Wherein, the chiral phosphoric acid substituent R is triphenylsilyl.
[0077] 1 H-NMR (500MHz, CDCl 3 ): 7.95(d, 2H), 7.62-7.65(m, 1H), 7.49-7.52(m, 2H), 7.28(d, J=8.7Hz, 2H), 7.23(d, J=8.7Hz, 2H) , 6.65(d, J=8.7Hz, 2H), 6.47(d, J=8.7Hz, 2H), 5.46-5.48(m, 1H), 4.78(d, J=10.1Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com