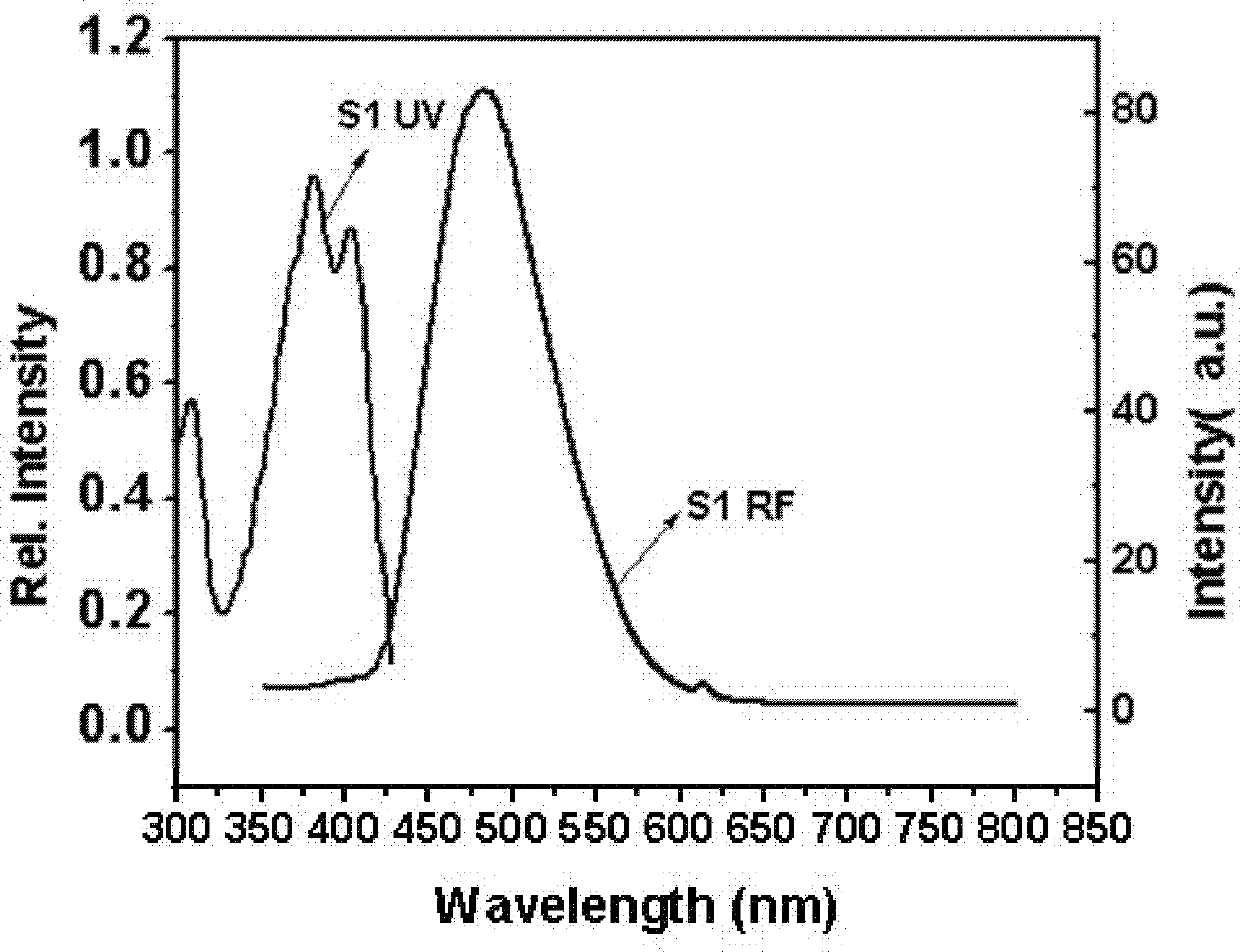
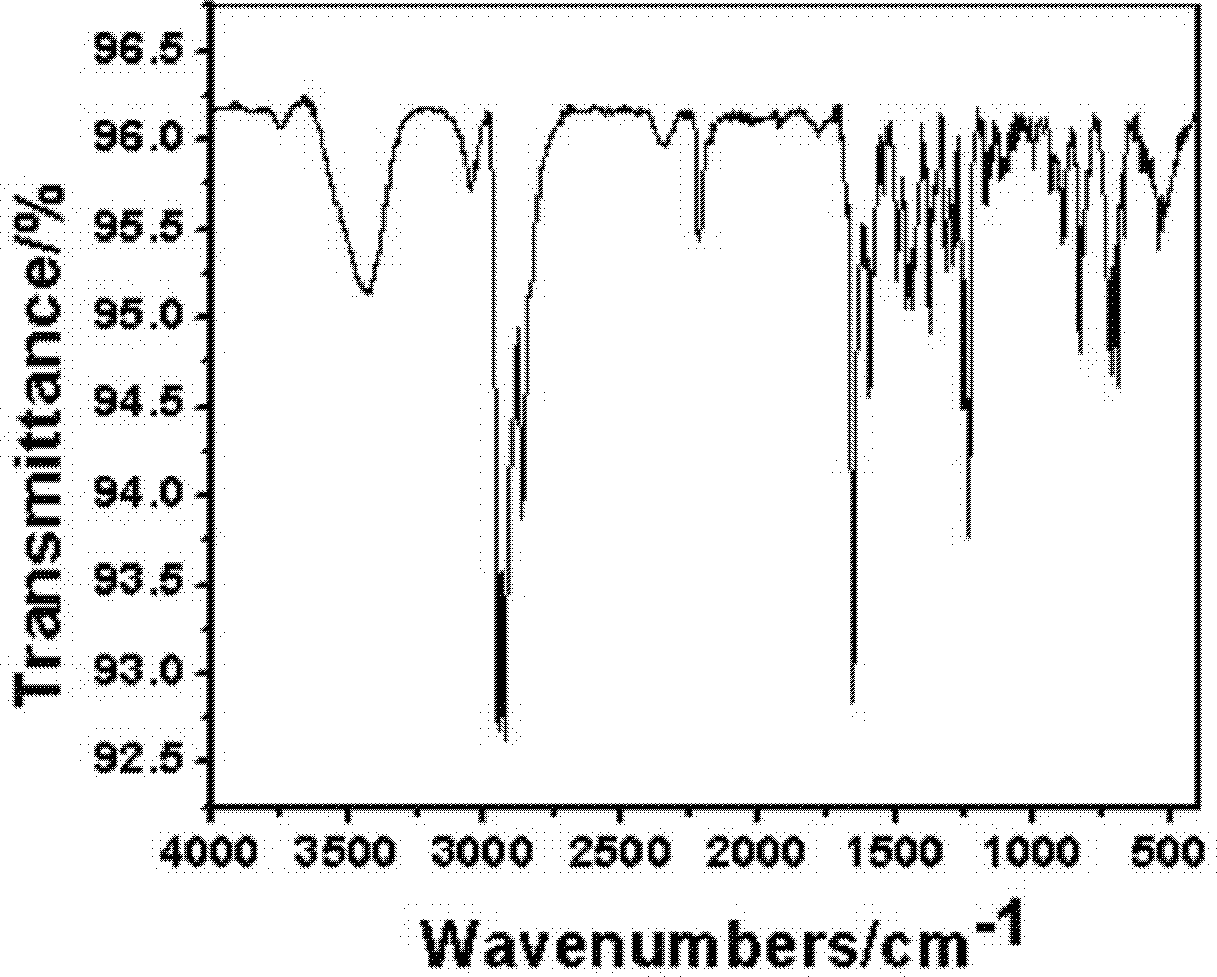
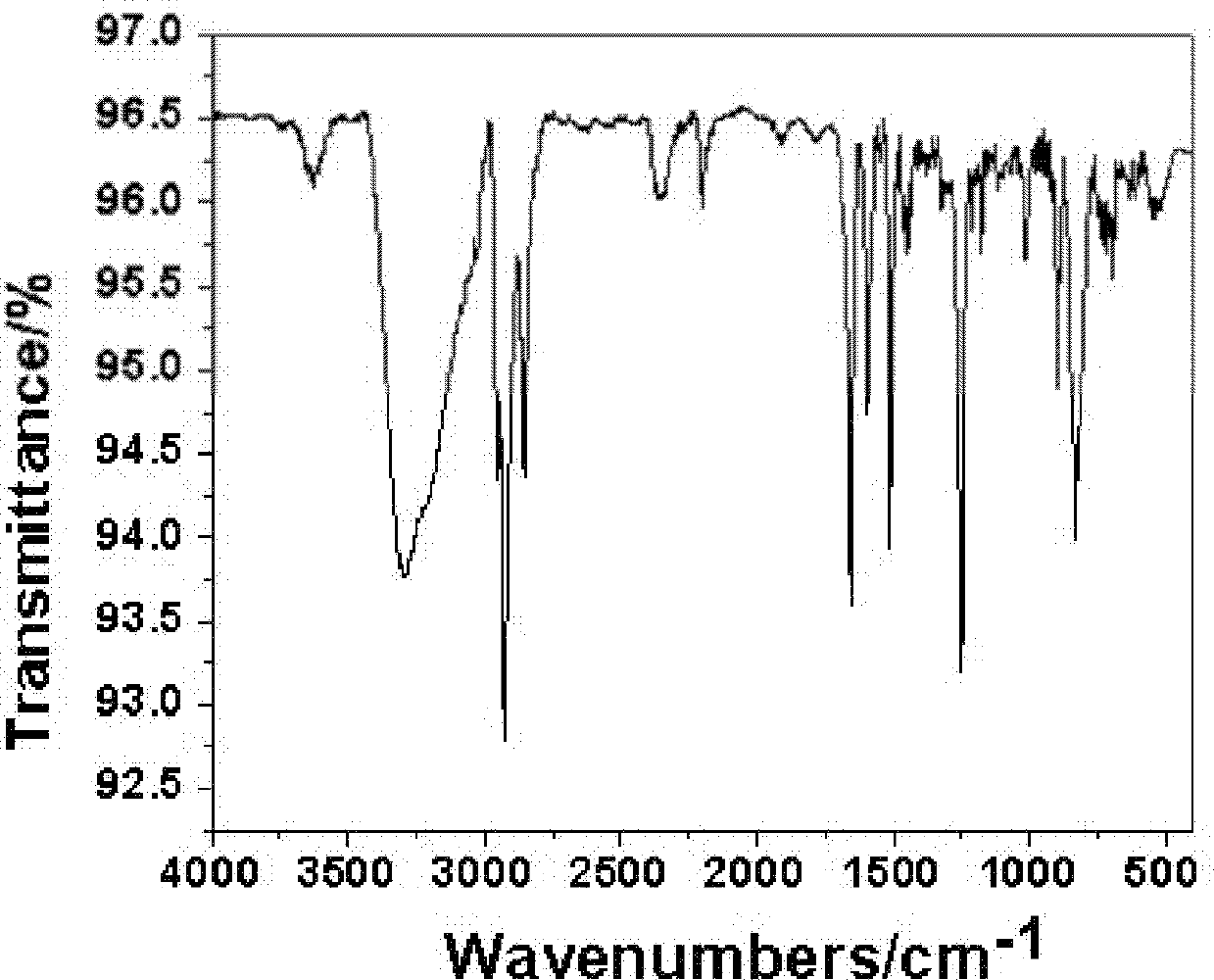
Pyrene type asymmetrical disc-like luminous compound and preparation method thereof
A luminescent compound and asymmetric technology, applied in the field of pyrene-based asymmetric biaxial discotic compounds and its preparation, and disc-shaped electroluminescent compounds, can solve the problems of no fluorescence, achieve strong fluorescence, high luminous efficiency, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] with R 1 structured as R 2 structured as
[0026] The synthetic method of this compound is introduced as an example
[0027] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide and place it in a 50mL single-necked bottle, add 1.5mmol p-bromobenzoyl chloride (3.26g), and cool to 0 in an ice bath degree, gradually add 0.02mol aluminum oxide (2.64g); heat and reflux for 14 hours; pour the product into 100mL ice water and stir for 2 hours after cooling, separate the liquid with dichloromethane, dry and filter the organic phase through anhydrous magnesium sulfate and reconstitute Crystallization gave 2.76 g of p-bromobenzoyl chloride monosubstituted pyrene, yellow needle-like crystals, and the yield was 72%.
[0028] 2: Intermediate product bromination reaction: 5mmol p-bromobenzoyl chloride monosubstituted pyrene (1.92g) obtained in step 1 was dissolved in 50mL nitrobenzene, placed in a 100mL single-necked bottle, and ...
Embodiment 2
[0033] with R 1 The structure is: with R 2 The structure is:
[0034] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide and put it in a 50mL single-necked bottle, add 1.5mmol p-bromobenzoyl chloride (3.26g), and place in an ice bath. After the reaction was stopped, the solvent was distilled off under reduced pressure, and the column developing solvent was a 1:1 mixed solvent of n-hexane and dichloromethane. 1.26 g of pale yellow product was obtained, yield 51%.
[0035] 4: Trimethylsilane removal reaction: Dissolve 1.28 g of 2 mmol of the intermediate product in step 3 in a mixed solution of 5 mL of methanol and 15 mL of tetrahydrofuran, place it in the reactor, add 2 g of potassium carbonate, stir at room temperature for 3 hours, and then depressurize The solvent was distilled off, washed with water and methanol; after vacuum drying for 5 hours, it was directly put into a 100mL two-necked bottle for the next reaction. ...
Embodiment 3
[0038] with R 1 The structure is: with R 2 The structure is:
[0039] The synthetic method of this compound is introduced as an example
[0040] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide and place it in a 50mL single-necked bottle, add 1.5mmol p-bromobenzoyl chloride (3.26g), and cool to 5mL in an ice bath degree, gradually add 0.02mol aluminum oxide (2.64g); heat and reflux for 12 hours; pour the product into 100mL ice water and stir for 4 hours after cooling, separate the liquid with dichloromethane, dry and filter the organic phase through anhydrous magnesium sulfate, and reconstitute Crystallization gave 2.76 g of p-bromobenzoyl chloride monosubstituted pyrene, yellow needle-like crystals, and the yield was 72%.
[0041] 2: Intermediate product bromination reaction: 5mmol p-bromobenzoyl chloride monosubstituted pyrene (1.92g) obtained in step 1 was dissolved in 50mL nitrobenzene, placed in a 100mL single-nec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com