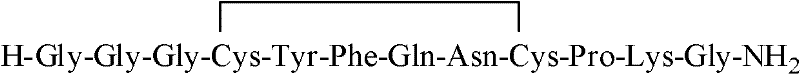Preparation method of Terlipressin
A technology of terlipressin and vasopressin, which is applied in the field of polypeptide drug preparation, can solve the problems of long air oxidation time, low total product yield, and reduced purity of crude products, etc., achieves extensive practical value and application prospects, and improves product quality. The effect of shortening the yield and production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthesis of embodiment 1Fmoc-Gly-Gly-Gly-OH
[0055] Take 3.0mol Fmoc-Gly and 3.0mol HOBt and dissolve them with appropriate amount of DMF; another 3.0mol DIC is slowly added to the protected amino acid DMF solution under stirring, and stirred and reacted at room temperature for 30 minutes to obtain the activated protected amino acid solution .
[0056] Take 1Kg of Fmoc-Gly-2-Cl-Trt-resin (substitution value is 1.0mmol / g), use 5L 20% PIP / DMF solution to remove Fmoc protection for 25 minutes, filter and wash the resin 3 times with MDF and DCM respectively, add The above-mentioned protected amino acid solution was stirred and reacted at room temperature for 3 hours. After the reaction was completed, the filtered resin was washed three times with MDF and DCM respectively.
[0057] The above two-step reaction was repeated, and another 2 Glys were added to obtain Fmoc-Gly-Gly-Gly-2-Cl-Trt-resin.
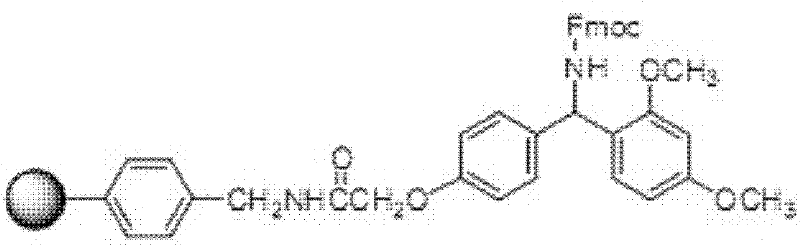
[0058] Take Fmoc-Gly-Gly-Gly-2-Cl-Trt-resin, add 20L 30% hexafluoroisop...
Embodiment 2
[0059] Example 2 Synthesis of Terlipressin Linear Peptide Resin
[0060] The terlipressin dendrimer is:
[0061] H-X-Cys(Trt)-Tyr(tBu)-Phe-Gln(Trt)-Asn(Trt)-Cys(Trt)-Pro-Lys(Boc)-Gly-resin wherein, X is Gly-Gly-Gly.
[0062] The Rink Amide AM resin was taken, and the terlipressin resin was obtained by sequentially coupling with the protected amino acids shown in Table 1 through de-Fmoc protection and coupling reactions. The protected amino acids used in this example correspond to the 1st to 10th amino acids from the resin as follows:
[0063] Table 1
[0064] The peptide sequence n=
Protected Amino Acids
molecular weight
1
Fmoc-Gly
297
2
Fmoc-Lys(Boc)
468
3
Fmoc-Pro
337
4
Fmoc-Cys(Trt)
586
5
Fmoc-Asn(Trt)
597
6
Fmoc-Gln(Trt)
611
7
Fmoc-Phe
387
8
Fmoc-Tyr(tBu)
460
9
Fmoc-Cys(Trt)
586
...
Embodiment 3
[0071] Example 3 Preparation of Crude Terlipressin Linear Peptide
[0072] Take the terlipressin linear peptide resin prepared in Example 2, add a cleavage reagent [TFA / water / EDT=95:5:5 (V / V) (10ml of cleavage reagent / g resin), stir evenly, and stir at room temperature React for 3 hours, filter the reaction mixture with a sand core funnel, collect the filtrate, wash the resin 3 times with a small amount of TFA, combine the filtrates, concentrate under reduced pressure, add anhydrous ether to precipitate, then wash the precipitate 3 times with anhydrous ether, and drain to obtain The white powder is the crude terlipressin linear peptide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com