Preparation method of iridium complex-containing phosphorescence material and its application in cobalt ion detection
A technology of iridium complexes and phosphorescent materials, applied in luminescent materials, fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of poor selectivity, high background, limited fluorescent probes and methods, etc., to achieve high selectivity, The effect of high sensitivity and high photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: The preparation steps of this embodiment are as follows:
[0036] (1) Preparation of 3, 4-diphenylcinnoline (dpci):
[0037] Dissolve 2g of 2-aminobenzophenone (10mmol) in 60mL of anhydrous diethyl ether, and then add dropwise therein the The Grignard reagent solution was refluxed for 45 minutes after the dropwise addition, and excess methanol and ammonium nitrate were added to quench the reaction. After washing with water and drying, the solvent was evaporated, and methanol recrystallized to obtain 2.7 g of light yellow prismatic crystal amino alcohol product, with a yield of 93%. m.p.149-150℃.
[0038] Add 0.75g (2.6mmol) of the product from the previous step into 20mL of 20% (volume ratio) sulfuric acid, heat at 100°C for 45min, adjust to weak alkalinity with aqueous ammonia, distill off the solvent after extraction with ether, add 5mL of acetic acid and 3.5 mL of concentrated hydrochloric acid, After the dissolution is complete, add 2.5% sodi...
Embodiment 2
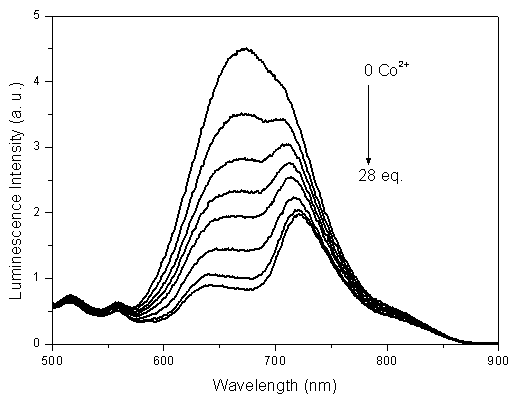
[0044] Embodiment 2: based on iridium complex Ir(dpci) 2 (dafbc)PF 6 Phosphorescence emission spectroscopy tests of phosphorescence chemical sensors responding to cobalt ions such as figure 1 Shown, in the mixed solution of dimethylformamide and water, Ir(dpci) 2 (dafbc)PF 6 The emission is pure red, and its maximum emission peak is at 673nm. With Co 2+ Gradually added, the intensity of the emission peak of 673 gradually weakened, while the peak at 721nm decreased slowly, and gradually emerged, and finally became the largest peak.
Embodiment 3
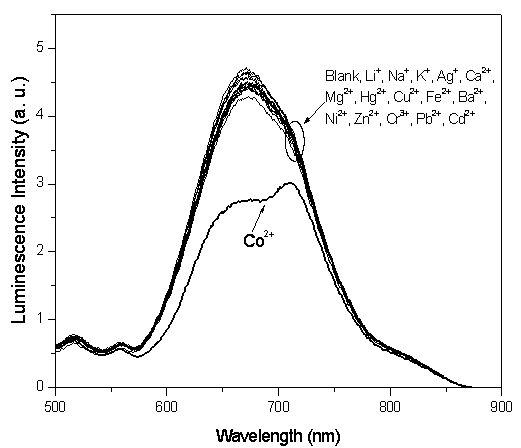
[0045] Embodiment 3: adding different metal ions (2.0 × 10 -4 mol / L), the iridium complex Ir(dpci) 2 (dafbc)PF 6 In dimethylformamide-water solution (1.0×10 -4 mol / L) changes in the emission spectrum such as figure 2 shown. It can be seen that the emission peak of the solution without adding metal ions is at 673nm; adding Li + , Na + , K + , Ca 2+ , Ba 2+ , Mg 2+ , Hg 2+ , Ag + , Cu 2+ , Fe 2+ , Ni 2+ , Zn 2+ , Cr 3+ , Pb 2+ and Cd 2+ After plasma, there is little change in peak intensity and no change in peak position. While joining Co 2+ The spectrum of the complex changed a lot, the peak at 673nm gradually weakened, and the peak at 721nm gradually appeared, and finally became the largest peak. This comparative experiment shows that Co 2+ The addition of complex Ir(dpci) 2 (dafbc)PF 6 The photophysical properties of the solution have obvious effects, and the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com