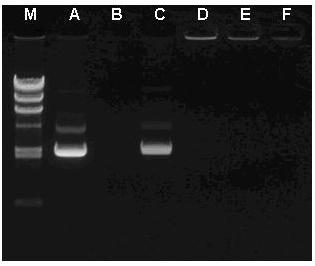
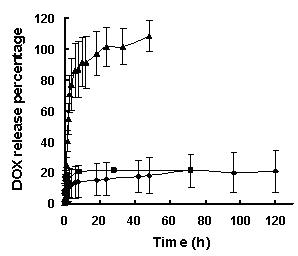
Tumor-targeting double-drug carrying and delivery system and preparation method thereof
A tumor-targeting, dual-drug-loading technology, which is applied in the field of biotechnology and pharmaceutical preparations, can solve the problems affecting tumor targeting efficiency and high concentration, and achieve the effects of high targeting and treatment efficiency, simple preparation, and small steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take 3 mg polyamide-amine (PAMAM, 77.35 mg / ml methanol solution) and dry it in a vial, add 0.73 mg polyethylene glycol (MAL-PEG3500-NHS, 1 mg / ml 0.035 M pH 8.0 phosphate solution) , the molar ratio of the two is 1:2, stirring and reacting at room temperature for 2 h, the NHS group reacts specifically with the amino group on the surface of PAMAM to make polyamide-amine-polyethylene glycol (PAMAM-PEG 2 ) complex solution, MWCO 10000 ultrafiltration centrifuge tube was ultrafiltered at 12000 rpm for 30 min to remove unreacted polyethylene glycol (PEG3500), and the synthesis of the carrier was characterized by hydrogen nuclear magnetic resonance.
Embodiment 2
[0040] Take 3 mg polyamide-amine (PAMAM, 77.35 mg / ml methanol solution) and dry it in a vial, add 0.73 mg polyethylene glycol (MAL-PEG3500-NHS, 1 mg / ml 0.035 M pH 8.0 phosphate solution) , the molar ratio of the two is 1:2, stirring and reacting at room temperature for 2 h, the NHS group reacts specifically with the amino group on the surface of PAMAM to make polyamide-amine-polyethylene glycol (PAMAM-PEG 2 ) complex solution, MWCO 10000 ultrafiltration centrifuge tube was ultrafiltered at 12000 rpm for 30 min to remove unreacted polyethylene glycol (PEG3500), reconstituted in pH 7.0 phosphate buffer, and added 0.10 mg polypeptide (T7, 2 mg / ml pH 7.0 phosphate solution), with a molar ratio of 1:1 to PAMAM, stirred at room temperature for 24 h, and the MAL group reacted specifically with the sulfhydryl group on the cysteine residue of the polypeptide (T7) to make a polyamide— Amine-polyethylene glycol-polypeptide (PAMAM-PEG-T7) complex, ultrafiltration in MWCO 10000 ultrafil...
Embodiment 3
[0042] Take an appropriate amount of doxorubicin methanol solution and dry it in a vial, add phosphate buffer with a pH value of 7.4 to prepare a 3.48 μg / ml doxorubicin solution, take 0.5 ml in a centrifuge tube, and use LS-55 fluorescence The spectrometer was excited at 480 nm, and the fluorescence emission spectrum of doxorubicin was detected in the range of 520-680 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com