First class s-triazine amphoteric betaine surfactant and synthesizing method thereof
A technology of surfactants and synthetic methods, applied in chemical instruments and methods, organic chemistry, transportation and packaging, etc., can solve problems such as human injury, and achieve the effects of not easy precipitation, shortened reaction time, and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
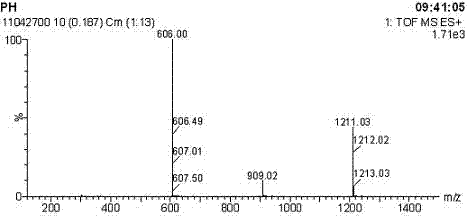
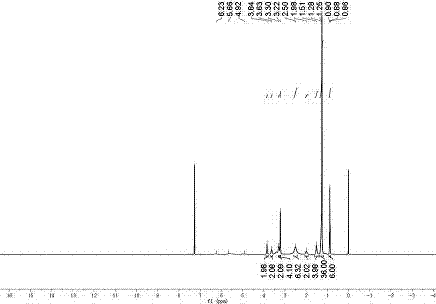
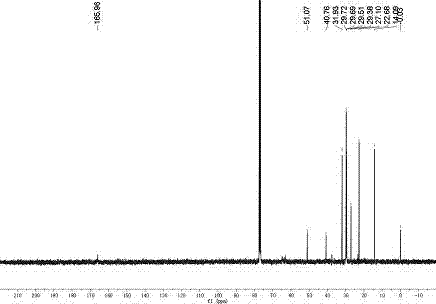
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of N-(4,6-bisoctylamino-1,3,5-s-triazin-2-yl)-N',N'-dimethyl-N'-carboxymethyl-1 ,2-Ethylenediamine amphoteric betaine surfactant
[0031] The first step reaction: the synthesis of intermediate 2,4-bisoctylamino-6-chloro-1,3,5-s-triazine
[0032] Dissolve 1.84g (0.01mol) of cyanuric chloride in 30mL of chloroform in a reaction flask, add dropwise a solution of 2.58g (0.02mol) of octylamine in chloroform at room temperature, and adjust the pH to 7~ with 5~8% potassium hydroxide solution. 10, reacted at room temperature for 12h. After the reaction was complete, the solvent was removed to obtain a white solid. The white solid was recrystallized from chloroform / methanol (1 / 8 to 1 / 1 by volume) to obtain 2.89 g of the intermediate 2,4-bis-octylamino-6-chloro-1,3,5-s-triazine. The rate is 78.3%.
[0033] The second step reaction: intermediate N-(4,6-bisoctylamino-1,3,5-s-triazin-2-yl)-N',N'-dimethyl-1,2-ethanedi Amine Synthesis
[0034] Stir 2.89g (...
Embodiment 2
[0037] Example 2: Preparation of N-(4,6-didecylamino-1,3,5-s-triazin-2-yl)-N',N'-dimethyl-N'-carboxymethyl-1 ,2-Ethylenediamine amphoteric betaine surfactant
[0038] The first step reaction: the synthesis of the intermediate 2,4-didecylamino-6-chloro-1,3,5-s-triazine
[0039] Dissolve 1.84g (0.01mol) of cyanuric chloride in 30mL of chloroform in a reaction flask, add dropwise a chloroform solution of 3.14g (0.02mol) of decylamine at room temperature, and adjust the pH to 7 to 7 with 5 to 8% sodium hydroxide solution. 10, reacted at room temperature for 12h. After the reaction was complete, the solvent was removed to obtain a white solid. The white solid was recrystallized from chloroform / methanol (1 / 8 to 1 / 1 by volume) to obtain 3.31 g of the intermediate 2,4-didecylamino-6-chloro-1,3,5-s-triazine. The rate is 77.8%.
[0040] The second step reaction: intermediate N-(4,6-didecylamino-1,3,5-s-triazin-2-yl)-N',N'-dimethyl-1,2-ethanedi Amine Synthesis
[0041] Stir 3.31g (...
Embodiment 3
[0044] Example 3: Preparation of N-(4,6-didodecylamino-1,3,5-s-triazin-2-yl)-N',N'-dimethyl-N'-carboxymethyl- 1,2-Propanediamine Amphoteric Surfactant
[0045] The first step reaction: the synthesis of the intermediate 2,4-didodecylamino-6-chloro-1,3,5-s-triazine
[0046] Dissolve 1.84g (0.01mol) of cyanuric chloride in 30mL of chloroform in a reaction flask, add dropwise a solution of 3.71g (0.02mol) of dodecylamine in chloroform at room temperature, and adjust the pH to 7 with 5-8% sodium hydroxide solution. ~10, reacted at room temperature for 12h. After the reaction was complete, the solvent was removed to obtain a white solid. The white solid was recrystallized from chloroform / methanol (1 / 8 to 1 / 1 by volume) to obtain 4.24 g of the intermediate 2,4-didodecylamino-6-chloro-1,3,5-s-triazine, The yield was 88.1%.
[0047] The second step reaction: intermediate N-(4,6-didodecylamino-1,3,5-s-triazin-2-yl)-N',N'-dimethyl-1,3-propane Synthesis of diamines
[0048] Stir 4.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com