Oral liquid composition for improving osteoarthritis and preparation method thereof
A technology of liquid composition and solution, which is applied in the direction of drug combination, medical preparations containing active ingredients, bone diseases, etc. It can solve the problems of aggregation and precipitation, difficulty in obtaining liquid preparations, etc., and achieve the effect of reliable quality and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
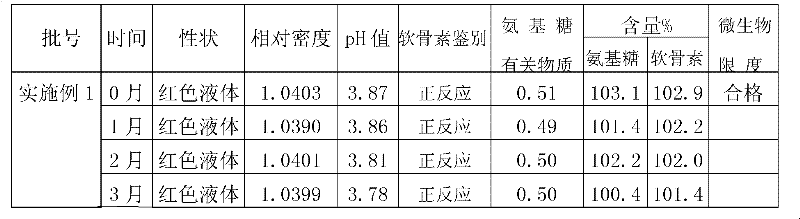
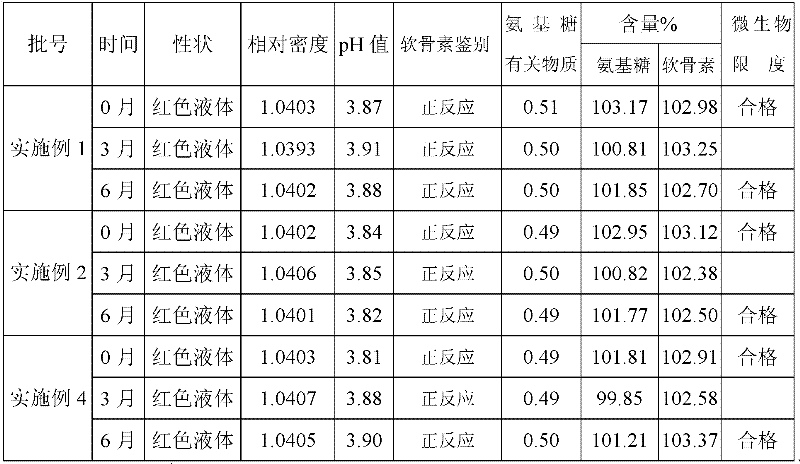
Examples
Embodiment 1
[0101] Embodiment 1: Glucosamine Hydrochloride Chondroitin Sulfate Oral Liquid (1000ml)
[0102] Prescription: Glucosamine Hydrochloride 50g
[0103] Chondroitin Sulfate 40g
[0104] Citric acid 3g
[0105] Stevioside 1g
[0106] Sodium Benzoate 1g
[0107] Potassium sorbate 1g
[0108] Sodium carboxymethylcellulose 0.3g
[0109] Grape essence 1ml
[0110] Carmine 60 0.15g
[0111] Distilled water 950ml
[0112] Preparation method: Dissolve the prescribed amount of chondroitin sulfate, citric acid, stevioside, sodium benzoate, potassium sorbate, and sodium carboxymethylcellulose in 950ml of distilled water, heat and boil for 30 minutes, cool to 30°C, add carmine After 60°C, nitrogen gas was introduced for 10 minutes, glucosamine hydrochloride and grape essence were added, and after stirring and dissolving completely, the pH and content of the intermediate were measured, filled with nitrogen, and sealed with a cover.
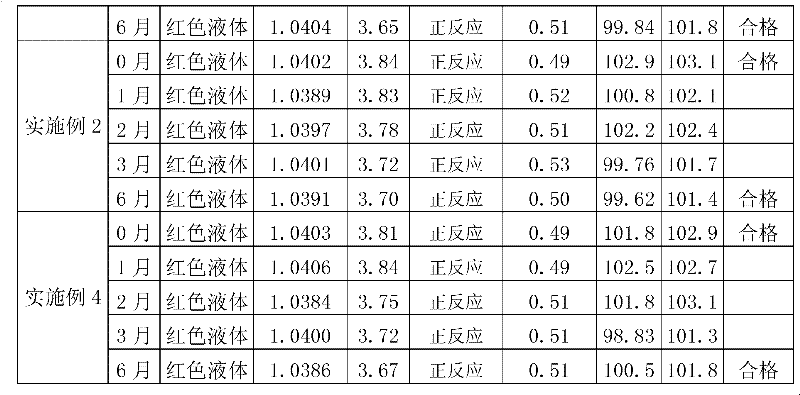
Embodiment 2
[0113] Embodiment 2: Glucosamine Hydrochloride Chondroitin Sulfate Oral Liquid (1000ml)
[0114] Prescription: Glucosamine Hydrochloride 40g
[0115] Chondroitin Sulfate 50g
[0116] Citric acid 3g
[0117] Stevioside 1g
[0118] Sodium Benzoate 1g
[0119] Potassium sorbate 1g
[0120] Sodium carboxymethylcellulose 0.3g
[0121] Grape essence 1ml
[0122] Carmine 60 0.15g
[0123] Distilled water 950ml
[0124] Preparation method: Dissolve the prescribed amount of chondroitin sulfate, citric acid, stevioside, sodium benzoate, potassium sorbate, and sodium carboxymethylcellulose in 950ml of distilled water, heat and boil for 30 minutes, cool to 30°C, add carmine After 60°C, nitrogen gas was introduced for 10 minutes, glucosamine hydrochloride and grape essence were added, and after stirring and dissolving completely, the pH and content of the intermediate were measured, filled with nitrogen, and sealed with a cover.
Embodiment 3
[0125] Embodiment 3: Glucosamine Hydrochloride Chondroitin Sulfate Oral Liquid (1000ml)
[0126] Prescription: Glucosamine Hydrochloride 60g
[0127] Chondroitin Sulfate 30g
[0128] Citric acid 3g
[0129] Stevioside 1g
[0130] Sodium Benzoate 1g
[0131] Potassium sorbate 1g
[0132] Sodium carboxymethylcellulose 0.3g
[0133] Grape essence 1ml
[0134] Carmine 60 0.15g
[0135] Distilled water 950ml
[0136] Preparation method: dissolve the prescribed amount of chondroitin sulfate, citric acid, stevioside, sodium benzoate, potassium sorbate, and sodium carboxymethylcellulose in 950ml of distilled water, heat and boil for 25 minutes, cool to 30°C, add carmine After 60°C, nitrogen gas was introduced for 10 minutes, glucosamine hydrochloride and grape essence were added, and after stirring and dissolving completely, the pH and content of the intermediate were measured, filled with nitrogen, and sealed with a cover.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com