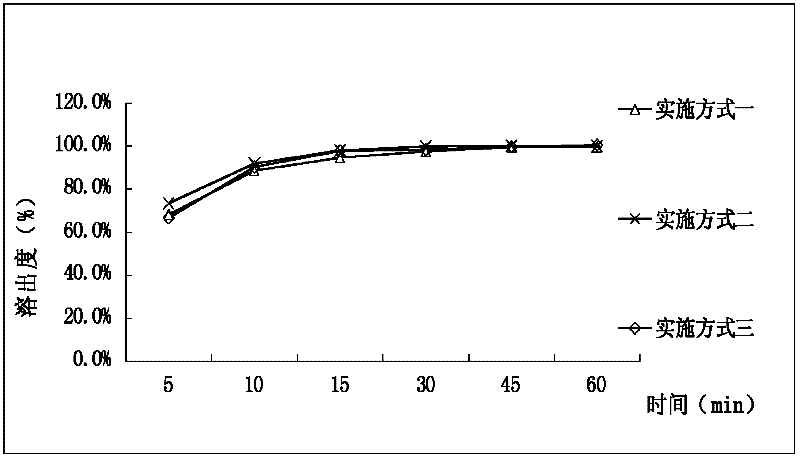
Cefetamet pivoxil hydrochloride capsule and preparation method thereof
A technology for preparing ceftamet pivoxil hydrochloride and capsules, which can be used in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as easy aggregation of ceftamet pivoxil hydrochloride, long process flow, and large amount of excipients , to achieve the effect of reducing agglomeration, low moisture, promoting dissolution and release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
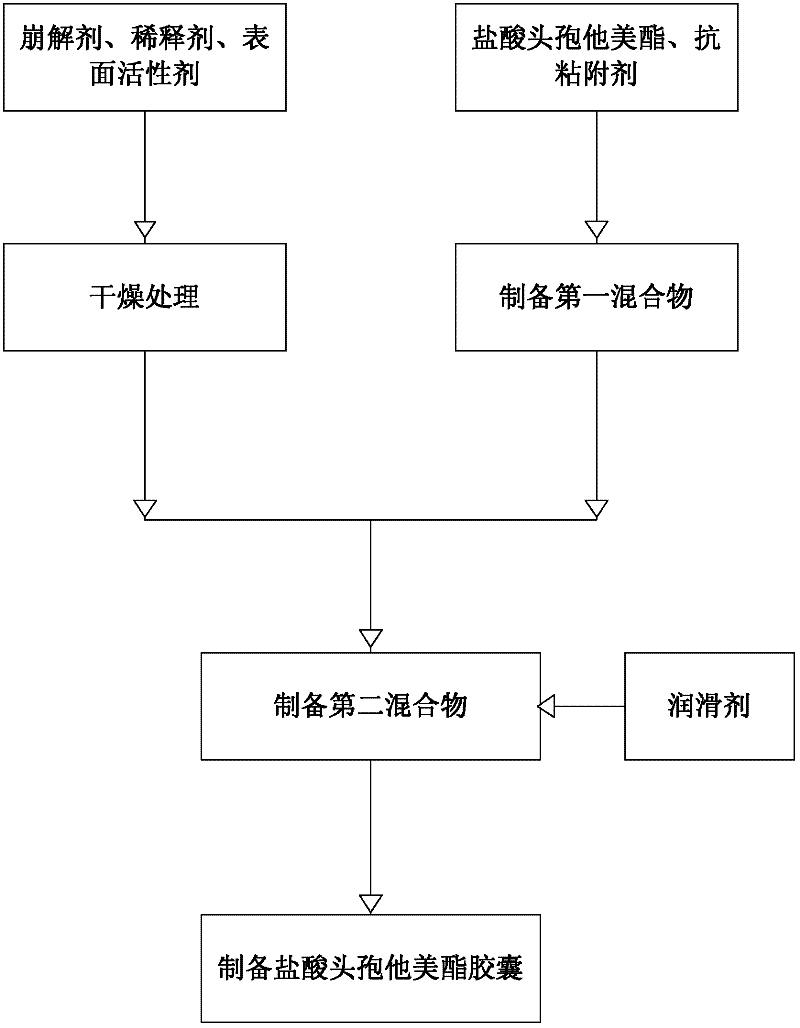
[0031] see figure 2 , figure 2 Show the embodiment of the present invention ceftazidime pivoxil hydrochloride capsule preparation method principle diagram, comprise the steps:
[0032] Step S01, drying process:
[0033] The components in the following weight percentages are dried separately:
[0034] Thinner 5~20%
[0035] Surfactant 0.1~4%
[0036] Disintegrant 2~10%;
[0037] Step S02, preparing the first mixture:
[0038] The components in the following weight percentages are mixed to obtain the first mixture:
[0039] Ceftazidime pivoxil hydrochloride 65~85%
[0040] Anti-adhesive agent 0.5-6%;
[0041] Step S03, preparing the second mixture:
[0042] mixing the first mixture, the dried diluent, surfactant and disintegrant, adding a lubricant with a weight percentage of 0.1 to 1%, and stirring to obtain a second mixture;
[0043] Step S04, preparing ceftazidime hydrochloride capsules:
[0044] The second mixture was filled into capsules to obtain ceftazidime h...
Embodiment 1
[0051] The ceftazidime hydrochloride capsule of the present embodiment one has the following components in weight percentage:
[0052]
[0053]
[0054] The preparation method of ceftazidime hydrochloride capsules of the present invention comprises the following steps:
[0055] (1), get cefetamet pivoxil hydrochloride 1212.18g, colloidal silicon dioxide N 20 62.3g, microcrystalline cellulose 272.18g, starch 50g, low-substituted hydroxypropyl cellulose 92.56g, crospovidone 71.2g, poloxamer 1.78g, magnesium stearate 17.8g;
[0056] (2), drying of auxiliary materials:
[0057] Dry the microcrystalline cellulose, starch, low-substituted hydroxypropyl cellulose, crospovidone and poloxamer in the above (1) at 105°C until the moisture content is 1%, and pass through a 80-mesh sieve respectively ;
[0058] (3), mixing:
[0059] Ceftazidime hydrochloride and colloidal silicon dioxide N in the above-mentioned (1) 20 Mix, cross 80 mesh sieves, obtain the first mixture;
[0060]...
Embodiment 2
[0064] The ceftazidime hydrochloride capsule of the present embodiment two has the following components in weight percentage:
[0065]
[0066]
[0067] The preparation method of ceftazidime hydrochloride capsules of the present invention comprises the following steps:
[0068] (1), get cefetamet pivoxil hydrochloride 1276.26g, colloidal silicon dioxide N 20 80.1g, microcrystalline cellulose 178g, pregelatinized starch 71.2g, crospovidone 129.94g, poloxamer 35.6g, talc 8.9g;
[0069] (2), drying of auxiliary materials:
[0070] Dry the microcrystalline cellulose, precrosslinked starch, crospovidone and poloxamer in the above (1) at 105° C. until the moisture content is 1%, and pass through 80-mesh sieve respectively;
[0071] (3), mixing:
[0072] Ceftazidime hydrochloride and colloidal silicon dioxide N in the above-mentioned (1) 20 Mix and pass through an 80-mesh sieve to obtain the first mixture;
[0073] (4), add microcrystalline cellulose, starch, low-substitut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com