Novel crystal form for temozolomide, method for preparing temozolomide and medicinal composition of temozolomide
A technology of temozolomide and its composition, which is applied in the direction of drug combination, medical preparations containing active ingredients, antineoplastic drugs, etc. It can solve the problems of being unsuitable for industrial production, difficult to meet the quality requirements of injection raw materials, and cumbersome operation, etc., to improve the appearance Color, suitable for long-term storage, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 Preparation of temozolomide M crystal form
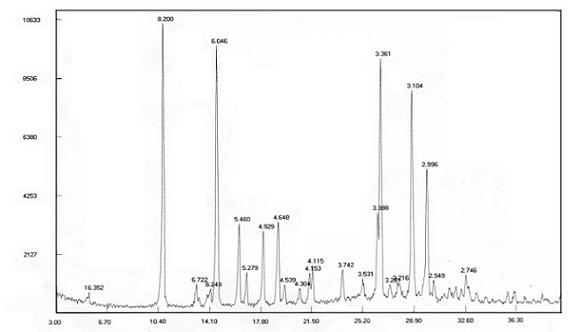
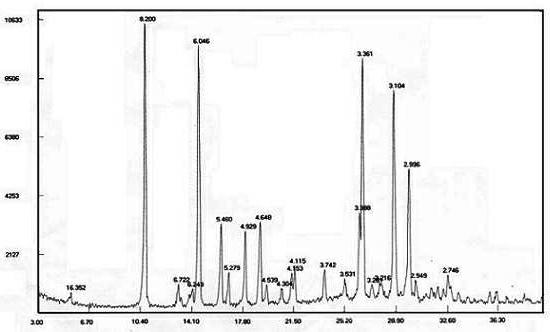
[0044]Take 10g of temozolomide and place it in a reaction flask, add 50g of acetone, 50g of acetonitrile and raise the temperature to 50-55°C while stirring, and slowly add 100g of purified water dropwise under stable reflux. With the continuous addition of purified water, the solid gradually dissolves. , slowly cooled to 30-40°C for crystallization for 1 hour, then crystallized at 15-25°C for 2 hours, and finally cooled to 5-10°C to fully separate out the solid and grow the crystal for 5 hours, then filter with suction, and wash the filter cake with water Wash with acetone / water (1:1) mixed solution and suck dry. The solid was dried at 45° C. under reduced pressure (-0.09 MPa) to obtain 8.1 g of white solid with a yield of 81%. ; Related substances 0.02%. By powder X-ray diffraction detection, such as figure 1 . It is the crystal form M of temozolomide.
[0045] HPLC purity testing method: take an appropria...
Embodiment 2
[0046] Embodiment 2 Preparation of temozolomide M crystal form
[0047] Take 10g of temozolomide and place it in a reaction flask, add 60g of acetone and 60g of acetonitrile, heat up to reflux under stirring, slowly add 40g of purified water dropwise under stable reflux, with the continuous addition of purified water, the solid gradually dissolves, after the dropwise addition, slowly Cool to 30-40°C to crystallize for 1 hour, then crystallize at 15-25°C for 2 hours, finally cool down to 5-10°C to fully separate out the solid and grow the crystal for 8 hours, filter with suction, rinse the filter cake with water and then use acetone / water (1:1) mixed solution was washed and drained. The solid was dried with phosphorus pentoxide as desiccant at 45° C. under reduced pressure (-0.09 MPa) to obtain 8.4 g of white solid with a yield of 84% and a purity of 100%. It was detected by powder X-ray diffraction that it was Temozolomide M crystal form.
Embodiment 3
[0048] Example 3 Preparation of Temozolomide M Crystal Form
[0049] Take 5 g of temozolomide and place it in a reaction flask, add 40 g of acetone and 20 g of acetonitrile, heat up to reflux under stirring, slowly add 30 g of purified water dropwise under stable reflux, and gradually dissolve the solid with the continuous addition of purified water. After the dropwise addition, slowly Cool to 30-40°C to crystallize for 1 hour, then crystallize at 15-25°C for 2 hours, finally cool down to 5-10°C to fully separate out the solid and grow the crystal for 10 hours, filter with suction, rinse the filter cake with water and then use acetone / water (1:1) mixed solution was washed and drained. The solid was dried with phosphorus pentoxide as desiccant at 45° C. under reduced pressure (-0.09 MPa) to obtain 4.3 g of white solid with a yield of 86% and a purity of 100%. It was detected by powder X-ray diffraction that it was Temozolomide M crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com