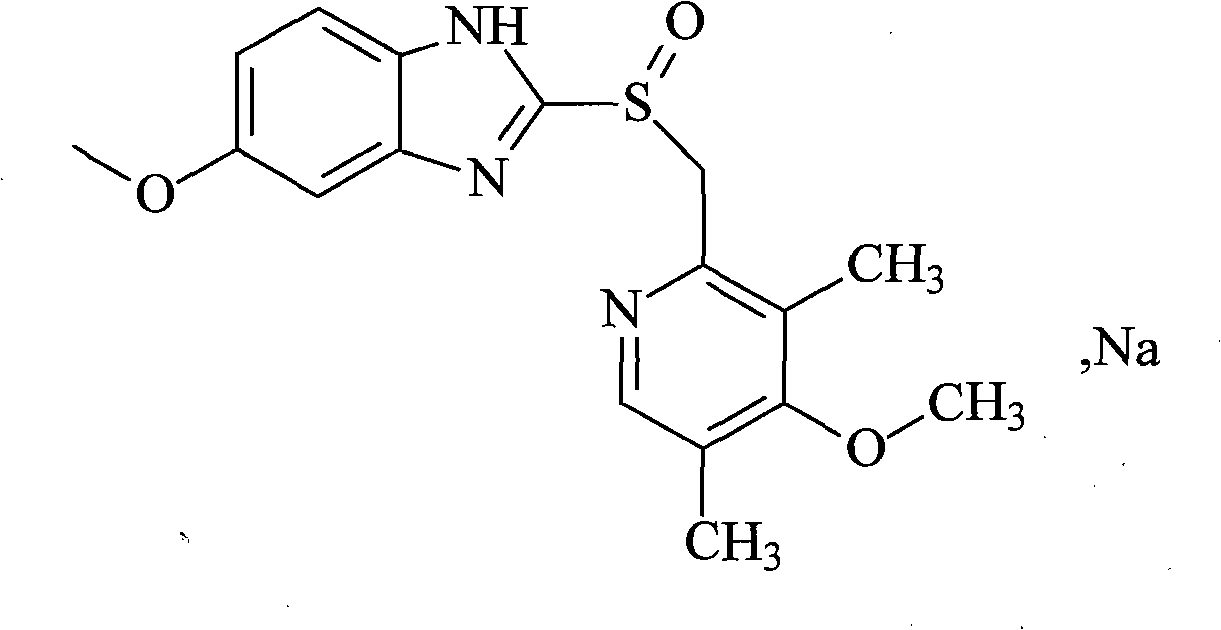
Esomeprazole sodium compound and preparation method thereof
A technology of esomeprazole sodium and compound, applied in the field of medicine, can solve the problems of low purity of esomeprazole sodium and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Get 10g crude esomeprazole sodium to be purified, the content of esomeprazole sodium recorded by high performance liquid chromatography is 92%. This esomeprazole sodium crude product is dissolved in 100ml water, fully stirs, and esomeprazole sodium is dissolved, crosses D1300 type macroporous adsorption resin, is the 1M sodium hydroxide solution elution purification of 12.5 with pH, collects containing esomeprazole sodium eluate.
[0053] Add 50 ml of 15% ammonia water to the above-mentioned aqueous eluting solution for treatment, the treatment time is 2 hours, stir during the treatment, and filter out the precipitated precipitate.
[0054] The obtained aqueous solution of esomeprazole sodium was raised to 60-70° C. to remove residual ammonia in the solution while concentrating to obtain a solution with a volume of 120 ml. Then add 75% ethanol to the aqueous solution, whose volume accounts for 65% of the aqueous solution volume, and slowly cool down to 12°C. Optiona...
Embodiment 2
[0057] Get 10g esomeprazole sodium crude product, the content of esomeprazole sodium recorded by high performance liquid chromatography is 93%. Dissolve the crude product of esomeprazole sodium in 120ml of water and stir fully, then dissolve the esomeprazole sodium, pass through BS-55 type macroporous adsorption resin, use pH 11.5 for elution and purification of 2M sodium carbonate solution, collect Eluate containing esomeprazole sodium.
[0058] Continuously feed ammonia gas into the above-mentioned aqueous eluting solution for treatment, the treatment time is 2.5 hours, stirring is carried out during the treatment, and the precipitated precipitate is filtered off.
[0059] Raise the obtained aqueous solution of esomeprazole sodium to 55-65°C, remove the remaining ammonia in the solution, and concentrate at the same time to obtain a solution volume of 90ml, then add absolute ethanol to the aqueous solution, the volume of which accounts for 1% of the aqueous solution The volu...
Embodiment 3
[0062] Get 10g of esomeprazole sodium crude drug (AstraZeneca AB, batch number: 20080503) with a longer production date, and the content of esomeprazole sodium recorded by high performance liquid chromatography is 91%. This esomeprazole sodium crude product is dissolved in 150ml water, after fully stirring, esomeprazole sodium is dissolved, cross BS-55 type macroporous adsorption resin, be 10 sodium bicarbonate solution elution purification with pH, The eluate containing esomeprazole sodium was collected.
[0063] Continuously feed ammonia gas into the above-mentioned aqueous eluting solution for treatment, the treatment time is 3 hours, stirring is carried out during the treatment, and the precipitated precipitate is filtered off.
[0064] The obtained esomeprazole sodium aqueous solution is raised to 70-80° C., removes the remaining ammonia in the solution, and concentrates at the same time to obtain a solution volume of 150 ml. Then, 90% ethanol is added to the aqueous solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com