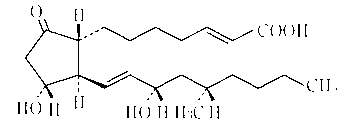
Limaprost nanoemulsion preparation for vertebral canal injection of antisternum
A technology of limaprost and spinal canal, which is applied in the field of pharmaceutical preparations, can solve the problems of short validity period, poor stability, and high storage conditions, and achieve the effects of overcoming low oral bioavailability, significant curative effect, and qualified pyrogen inspection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
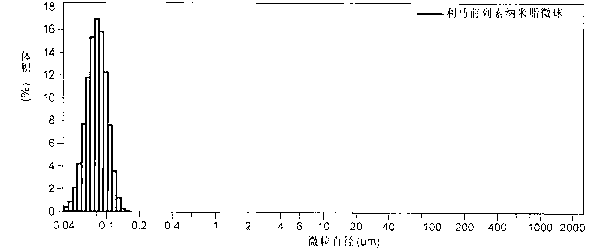
[0042] Preheat 60g of soybean oil for injection to 65°C, add 10g of refined soybean lecithin and stir with hand to form a uniform oil phase, then slowly add 3mg of limaprost raw material drug, stir at 70°C at high speed (8000 rpm) for 3 minutes, Dissolve it evenly in the oil phase; dilute 20g of glycerin for injection, 35mg of emulsion stabilizer sodium citrate, and 35mg of emulsion stabilizer citric acid with an appropriate amount of water for injection preheated to 60~70°C to form a uniform water Phase; pour the above water phase into the mixer, slowly drop the oil phase into the water phase under the stirring condition of 10000 rpm, stir for 10 minutes, and form milky white colostrum after uniform dispersion; add the colostrum to Preheat to 60~70℃ water for injection to reach the full amount, transfer to a high-pressure homogenizer, homogenize 10 times under 8000 Psi pressure, take a sample to measure the particle size until the average particle size is below 200 nanometers,...
Embodiment 2
[0044]Preheat 70g of soybean oil for injection to 60~70°C, add 15g of refined lecithin with hand-held stirring to form a uniform oil phase, then slowly add 4mg of limatoprost raw material drug, stir at 70°C at high speed (8000 rpm) 4 Minutes to make it evenly dissolve in the oil phase; Dilute 10g of glycerin for injection, 25mg of emulsion stabilizer sodium citrate, 25mg of emulsion stabilizer citric acid with an appropriate amount of water for injection preheated to 60~70℃ to form a uniform The water phase; the above water phase is poured into the mixer, the oil phase is slowly dropped into the water phase under the stirring condition of 10000 rpm, stirred for 10 minutes, and milky white colostrum is formed after uniform dispersion; the colostrum Add it to the water for injection preheated to 60~70°C to reach the full amount, transfer it to a high-pressure homogenizer, homogenize it under 15000 Psi pressure for 8 times, take a sample to measure the particle size until the aver...
Embodiment 3
[0046] Preheat 50g of hydrogenated corn oil to 69°C, add 15g of polyoxyethylene castor oil and stir with hand to form a uniform oil phase, then slowly add 6mg of limaprost raw material drug, stir at 70°C at high speed (8000 rpm) for 3 minutes , so that it is uniformly dissolved in the oil phase; dilute 30g of glycerin for injection, 50mg of emulsion stabilizer sodium citrate, and 50mg of emulsion stabilizer citric acid with an appropriate amount of water for injection preheated to 60~70°C to form a uniform Water phase: pour the above water phase into the mixer, slowly drop the oil phase into the water phase under the stirring condition of 9000 rpm, stir for 12 minutes, and form milky white colostrum after uniform dispersion; add the colostrum Add the full amount to the water for injection preheated to 60~70°C, transfer to the high-pressure homogenizer, homogenize 10 times under the pressure of 10,000 Psi, take samples to measure the particle size until the average particle size...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com