Low-temperature reduction type cobalt (Co)-based Fischer-Tropsch synthesis catalyst and preparation method thereof
A catalyst and reduction type technology, applied in the field of low temperature reduction type cobalt-based Fischer-Tropsch synthesis catalyst and its preparation, can solve the problems of dangerous storage and use, easy combustion, complicated activation treatment steps, etc. The effect of good Fischer-Tropsch reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 219.31g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 852g distilled water to make Zn(NO 3 ) 2 Mass concentration 15% zinc nitrate aqueous solution, 102.0g (NH 4 ) 2 CO 3 Dissolve in 531g distilled water to make 16% ammonium carbonate aqueous solution with mass concentration. Zinc nitrate aqueous solution and ammonium carbonate aqueous solution are simultaneously pumped into the sedimentation tank for co-precipitation reaction, the precipitation temperature is 50±2°C, the pH value is controlled at 6.2±0.2, and the reaction time is 36 minutes. The obtained precipitate was washed with distilled water until there was no ammonia odor, filtered, and the filter cake was dried at 120°C overnight, and then calcined at 500°C for 6 hours to obtain 60g of ZnO carrier, which was named carrier 1 # .
Embodiment 2
[0033] 73.1g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 250g distilled water to make Zn(NO 3 ) 2 Mass concentration 17% zinc nitrate aqueous solution, 35.42g (NH 4 ) 2 CO 3 Dissolve in 177g distilled water to make 17% ammonium carbonate aqueous solution with mass concentration. Zinc nitrate aqueous solution and ammonium carbonate aqueous solution are simultaneously pumped into the sedimentation tank for co-precipitation reaction, the precipitation temperature is 55±2°C, the pH value is controlled at 6.4±0.2, and the reaction time is 13 minutes. The obtained precipitate was washed with distilled water until there was no ammonia smell, filtered, and the filter cake was dried at 120°C overnight, and then calcined at 500°C for 6 hours to obtain 20g of ZnO carrier, which was named carrier 2 # .
Embodiment 3
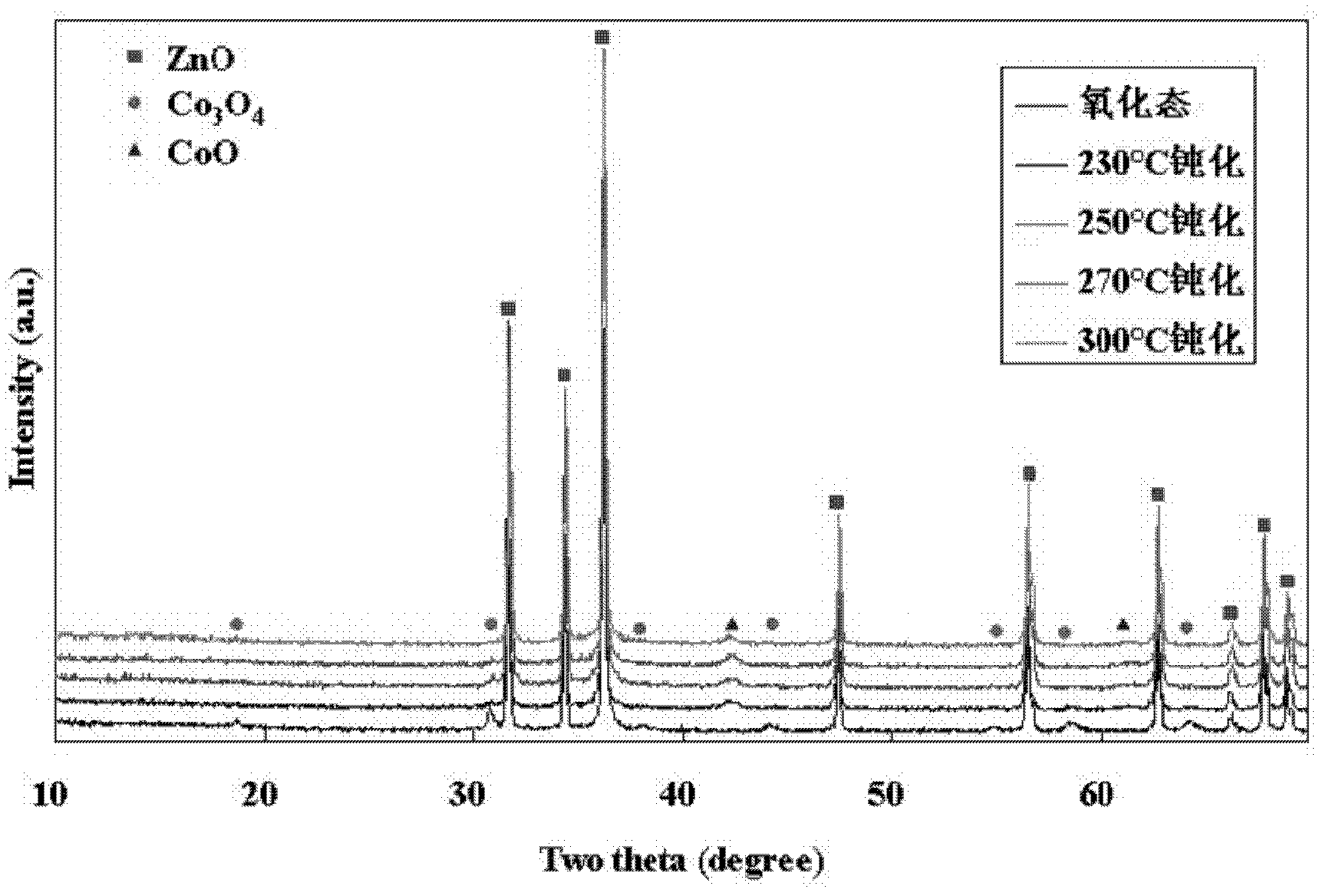
[0035] 2.6g Co(NO 3 ) 2 ·6H 2 O was dissolved in 4.0g distilled water to make Co(NO 3 ) 2 Mass concentration 33% cobalt nitrate aqueous solution, the carrier 1 prepared in embodiment 1 # Shaped into 150-280μm particles, take 10.0g ZnO and put it into the above-mentioned cobalt nitrate aqueous solution, impregnate at 20℃ for 10h, put the carrier and impregnated liquid together in an oven at 120℃ to dry overnight, and then bake at 500℃ for 6h to obtain Co / ZnO catalyst , named Catalyst 3 # , the mass percentage of the active component Co of the catalyst is 5wt%.
[0036] Get respectively the catalyst 3 prepared by the above-mentioned method of 0.5g # Put it into a fixed-bed reactor (ZJGD-XJ2009-041, Tianjin Pengxiang Technology Co., Ltd.) for reduction with pure hydrogen. The reduction temperatures are 230°C, 250°C, 270°C and 300°C, and the reduction time is 6 hours. After the reduction Lower the temperature to 150°C in hydrogen, then switch to nitrogen purging for 0.5h, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com