Novel quinone compound and preparation method thereof
A compound and quinone technology, applied in the field of new organic compounds and their preparation, can solve the problems of low theoretical specific capacity, limited raw material reserves, environmental pollution by heavy metal elements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
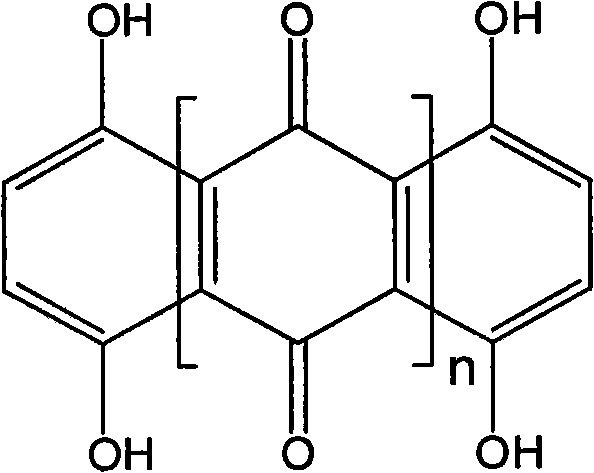
[0018] Dissolve 0.6 g of 1,4,5,8-tetrahydroxy-9,10-anthraquinone (THAQ) in 160 mL of acetonitrile, then add 6.0 g (NH 4 ) 2 S 2 o 8 , The stirring reaction was continued at room temperature for 16h. Afterwards, the product was filtered, washed with deionized water, and finally washed with acetone until the filtrate was colorless. The obtained dimer product (THHQ) was vacuum-dried at room temperature for 16 h to obtain a black solid powder with a yield of about 80%.
example 2
[0020] Add 0.6g of 1,4,5,8-tetrahydroxy-9,10-anthraquinone (THAQ) into 160mL of ethyl acetate, heat to boiling and add 10.0g of FeCl 3 , Continuous reflux reaction for 20h. The product was then filtered and washed with deionized water to be free of Cl - (with AgNO 3 test), and finally washed with acetone until the filtrate was colorless. The obtained product was vacuum-dried at room temperature for 16 hours to obtain a black solid powder with a yield of about 70%.
example 3
[0022] Add 1.0g of 1,4,5,8-tetrahydroxy-9,10-anthraquinone (THAQ) into 160mL of N,N-dimethylformamide, heat to boil and dissolve it, add 5.0g of KClO 3 , Continuous reflux reaction for 5h. After cooling to room temperature, the product was filtered, washed with deionized water, and finally washed with acetone until the filtrate was colorless. The obtained product was vacuum-dried at room temperature for 16 hours to obtain a black solid powder with a yield of about 75%.
[0023] figure 1 It is the FT-IR spectrum of dimer THHQ (a) and THAQ (b): both are at 3424cm -1 The strong absorption peak of represents the -OH stretching vibration. 1589cm -1 、1598cm -1 They are respectively the stretching vibration peaks of C=O on the aromatic rings of THHQ and THAQ. Due to the enhancement of the conjugation effect, the infrared absorption peak of the group can be blue-shifted, which proves that THHQ has a larger conjugated system than THAQ molecules. For the same reason, the stretchin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com