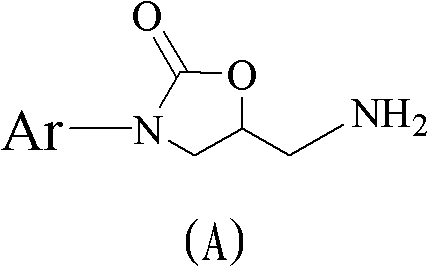
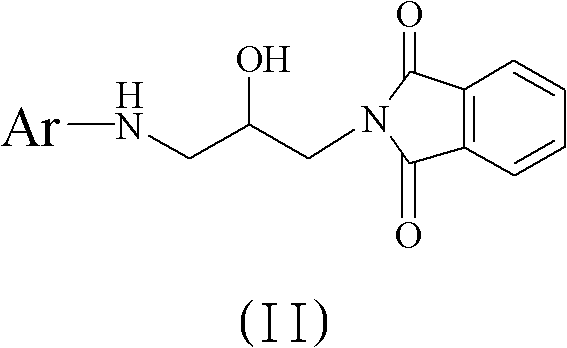
Method for preparing 5(S)-aminomethyl-3-aryl-2-oxazolidinone
A technology of oxazolidinones and aminomethyl groups, which is applied in the field of preparation of 5-L-aminomethyl-3-aryl-2-oxazolidinones, and can solve problems affecting optical purity, long reaction time, and temperature It is not suitable for problems such as excessively high, and achieves the effects of low cost, high total yield, and easy recovery and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
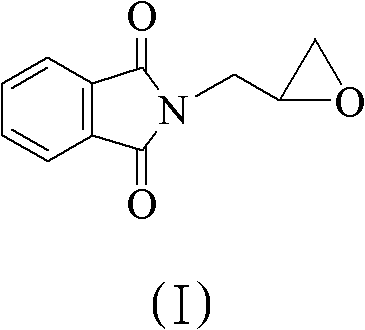
[0025] The preparation method of L-glycidylphthalimide ((S)-glycidylphthalimide) (I):
[0026]Weigh potassium phthalimide (Potassium phthalimide) (8.01g, 43.25mmol) and place it in a double-neck flask, add (S)-Epichlorohydrin (10ml, 127.5mmol) and tetrabutyl Ammonium bromide (1.2g, 3.727mmol) (suspended as light yellow solid) was reacted at 30°C for 5.5 hours. After the reaction was finished, add 80ml of water and 80ml of ethyl acetate, extract, separate layers, extract the organic layer with 320ml of saturated brine, and dissolve with anhydrous magnesium sulfate (MgSO 4 ) was dried, filtered, and the filtrate was concentrated to dryness, and the resulting solid was dried with oil-free pumping for 16 hours to obtain 7.3 g of solid L-glycidyl phthalimide (I). The optical purity was 87% ee.
[0027] 1 H-NMR (deuterated chloroform, CDCl 3 ):
[0028] 2.65(dd, J=4.8Hz, 2.5Hz, 1H), 2.78(dd, J=4.8Hz, 2.5Hz, 1H), 3.21(m, 1H), 3.78(dd, J=14.4Hz, 5.0Hz, 1H ), 3.93(dd, J=14.4Hz, 5...
Embodiment 2
[0039] The preparation method of L-glycidyl phthalimide (I): embodiment is the same as Example 1, only benzyltrimethylammonium chloride is replaced with tetrabutylammonium bromide to obtain 7.5g solid L-phthalimide Oxypropylphthalimide (I). The optical purity is 98% ee.
Embodiment 3
[0041] The preparation method of L-glycidyl phthalimide (I): the embodiment is the same as in Example 1, only benzyltrimethylammonium chloride is replaced with tetrabutylammonium bromide, and the reaction solvent is Virahol simultaneously 7.7 g of solid L-glycidylphthalimide (I) were obtained. The optical purity is 98.3%ee.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com