Hydrocracking catalyst and preparation method thereof
A hydrocracking and catalyst technology, applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem of poor stability and insignificant improvement in catalyst stability, The problem of increased diffusion resistance of reactants and products, etc., achieves the effects of good stability, improved conversion rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Select the carrier: the carrier is 40-60 mesh, the specific surface is 220m 2 / g of alumina.
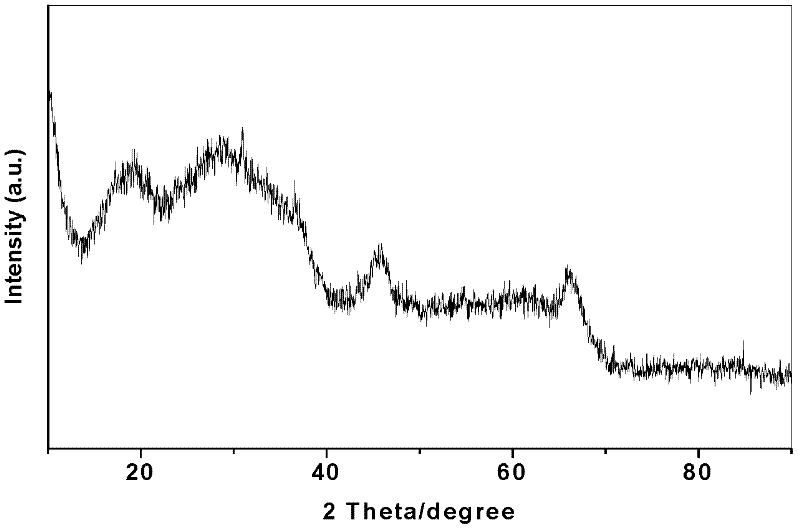
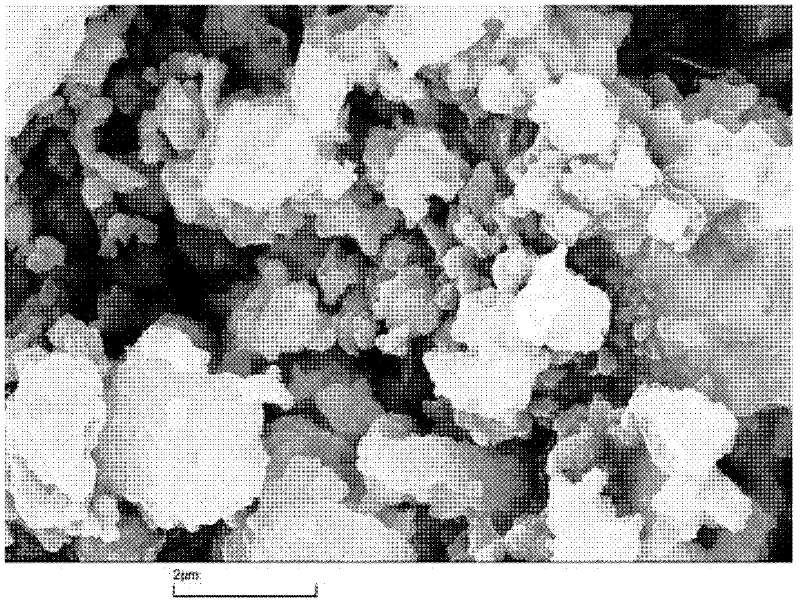
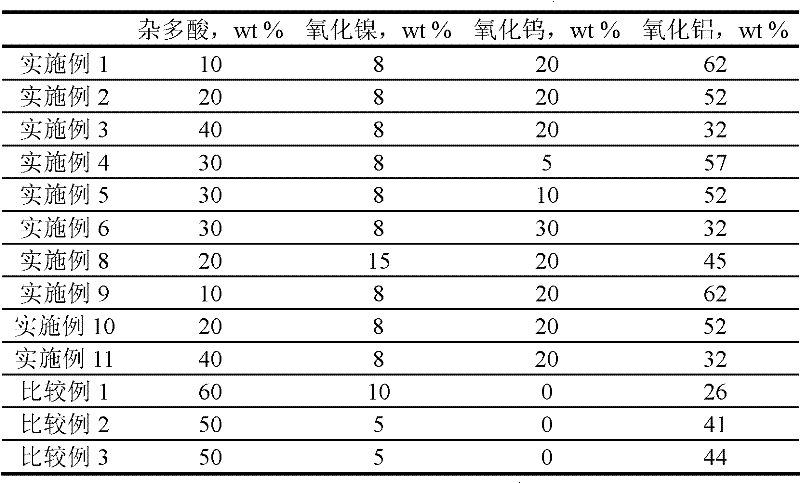
[0023] 2) Catalyst preparation: 110ml of nickel nitrate solution with a concentration of 1mol / L and 86ml of ammonium metatungstate solution with a concentration of 1mol / L were added to 62g of the above carrier Al 2 O 3 In the middle, let stand for 10 hours, and then dry at 110 ℃ for 5 hours. The dried solid was raised to 550°C at a rate of 10°C / min in a muffle furnace and kept at a constant temperature for 4h. The calcined solid was immersed in 10 g of phosphotungstic acid aqueous solution, immersed for 8 hours and then dried at 110° C. for 6 hours, and the dried material was pressed into tablets to obtain the catalyst. The chemical composition of the prepared catalyst is listed in Table 1.
[0024] 3) Evaluation conditions: set the prepared catalyst at 5 / 95 (volume ratio) CS 2 / H 2 Vulcanize as sulfide gas at 300°C for 4h. After the vulcanization is completed, cut into hydrog...
Embodiment 2
[0026] 1) Select the carrier: the carrier is 40-60 mesh, the specific surface is 220m 2 / g of alumina.
[0027] 2) Catalyst preparation: 110ml of nickel nitrate solution with a concentration of 1mol / L and 86ml of ammonium metatungstate solution with a concentration of 1mol / L were added to 52g of Al 2 O 3 In the middle, let stand for 10 hours, and then dry at 110 ℃ for 5 hours. The dried solid was raised to 550°C at a rate of 10°C / min in a muffle furnace and kept at a constant temperature for 4h. The calcined solid was immersed in 20 g of phosphotungstic acid aqueous solution, immersed for 8 hours and then dried at 110° C. for 6 hours, and the dried material was pressed into tablets to obtain the catalyst. The chemical composition of the prepared catalyst is listed in Table 1.
[0028] 3) Evaluation conditions: set the prepared catalyst at 5 / 95 (volume ratio) CS 2 / H 2 Vulcanize as sulfide gas at 300°C for 4h. After the vulcanization is completed, cut into hydrogen, and enter n-de...
Embodiment 3
[0030] 1) Select the carrier: the carrier is 40-60 mesh, the specific surface is 220m 2 / g of alumina.
[0031] 2) Catalyst preparation: 110ml of nickel nitrate solution with a concentration of 1mol / L and 86ml of ammonium metatungstate solution with a concentration of 1mol / L were added to 32g of the above carrier Al 2 O 3 In the middle, let stand for 10 hours, and then dry at 110 ℃ for 5 hours. The dried solid was raised to 550°C at a rate of 10°C / min in a muffle furnace and kept at a constant temperature for 4h. The calcined solid was immersed in an aqueous solution containing 40 g of phosphotungstic acid, immersed for 8 hours and then dried at 110° C. for 6 hours, and the dried material was pressed into tablets to obtain the catalyst. The chemical composition of the prepared catalyst is listed in Table 1.
[0032] 3) Evaluation conditions: set the prepared catalyst at 5 / 95 (volume ratio) CS 2 / H 2 Vulcanize as sulfide gas at 300°C for 4h. After the vulcanization is completed, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com