Renaturing and purifying method of mycobacterium-tuberculosis recombinant inclusion-body protein
A technology of Mycobacterium tuberculosis and purification method, which is applied in the field of renaturation and purification of recombinant inclusion body protein of Mycobacterium tuberculosis, can solve the problems of many process steps, difficult quality control, high price, etc., and achieve less process steps and short lifespan , Ease of quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
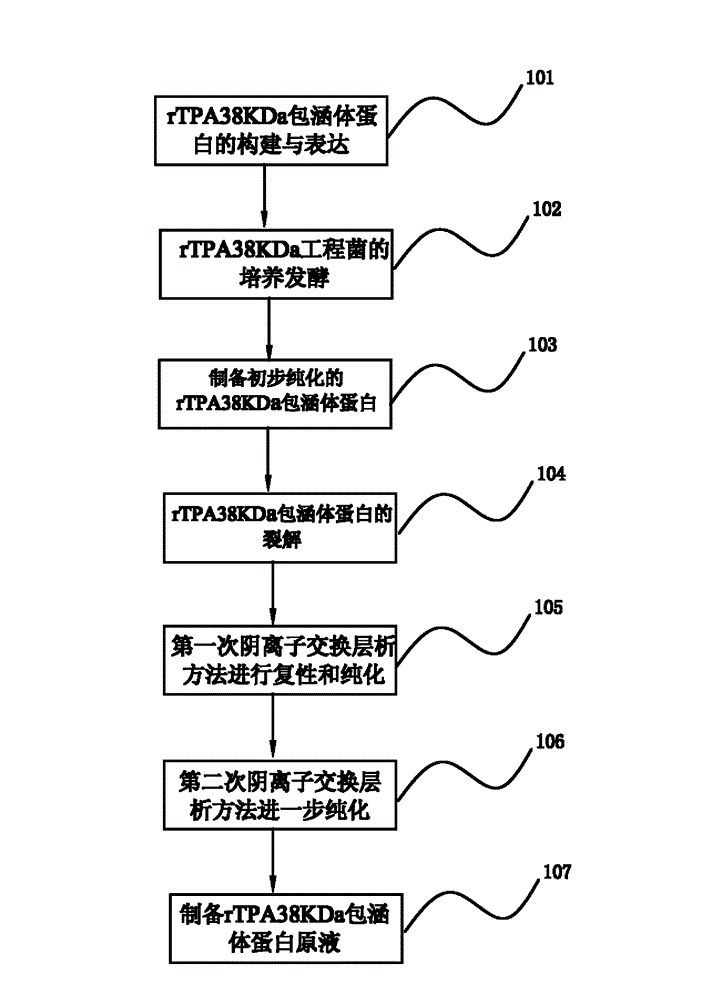
[0058] Example 1 of the renaturation and purification method of the Mycobacterium tuberculosis recombinant inclusion body protein of the present invention figure 1 shown, including the following steps:
[0059] Step 101. Construction and expression of rTPA38KDa inclusion body protein
[0060] The engineering bacteria used in the present invention: the recombinant Mycobacterium tuberculosis 38KDa protein (rTPA38KDa) strain is Escherichia coli BL21 (DE3) transformed by the PET9d-Pad plasmid carrying the 38KDa protein coding gene (Pab) of the Mycobacterium tuberculosis H37Rv strain The strain was constructed and provided by the 309 Hospital of the People's Liberation Army.
[0061] The engineering bacterium is Escherichia coli containing recombinant Mycobacterium tuberculosis 38KDa protein (rTPA38KDa) expression vector PET9d-TPA38. PET9d-TPA38 contains the rTPA38KDa coding sequence, and the target gene is placed under the control of the strong promoter T7 promoter. Under the ...
Embodiment 2
[0105] A specific example of the renaturation and purification method of a Mycobacterium tuberculosis recombinant inclusion body protein of the present invention is as follows: figure 1 As shown, the main technical solutions of this embodiment are the same as those of Embodiment 1, and the features not explained in this embodiment are explained in Embodiment 1, and will not be repeated here. The difference between this embodiment and embodiment 1 is:
[0106] A method for renaturation and purification of Mycobacterium tuberculosis recombinant inclusion body protein of the present invention comprises the following steps:
[0107] Step 101. Construction and expression of rTPA38KDa inclusion body protein
[0108] This step is the same as in Example 1.
[0109] Step 102. Cultivation and fermentation of rTPA38KDa engineering bacteria
[0110] Inoculate the engineering bacteria glycerol strain into the Erlenmeyer flask containing LB medium at an inoculation ratio of 1% (V / V), an...
Embodiment 3
[0155] A specific example of the renaturation and purification method of a Mycobacterium tuberculosis recombinant inclusion body protein of the present invention is as follows: figure 1 As shown, the main technical solutions of this embodiment are the same as those of Embodiment 1, and the features not explained in this embodiment are explained in Embodiment 1, and will not be repeated here. The difference between this embodiment and embodiment 1 is:
[0156] A method for renaturation and purification of Mycobacterium tuberculosis recombinant inclusion body protein of the present invention comprises the following steps:
[0157] Step 101. Construction and expression of rTPA38KDa inclusion body protein
[0158] This step is the same as in Example 1.
[0159] Step 102. Cultivation and fermentation of rTPA38KDa engineering bacteria
[0160] Inoculate the engineering bacteria glycerol strains into the Erlenmeyer flask containing LB medium at the inoculation ratio of 1% (V / V), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com