Preparation method of urapidil by reverse phase transfer catalysis
A catalytic preparation, urapidil technology, applied in the direction of chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of not providing product purity and difficult to achieve pharmaceutical purity and other problems, to achieve the effects of easy control of reaction conditions, shortened reaction time, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1-(2-Methoxyphenyl)piperazine hydrochloride (5.67g, 0.025mol) was added to Na 2 CO 3 (5.27g, 0.050mol) and the mixture of 25ml water, be heated to 90 ℃, add 6-(3-chloropropyl)-1,3-dimethyluracil (5.00g, 0.022mol) in batches, Then add 0.2 g of β-cyclodextrin, complete the addition, react for 6 hours, add water, stir and cool, a white substance precipitates, filter under reduced pressure, and dry at room temperature to obtain 6.91 g of white powdery solid, with a yield of 82.6% and a purity of 99.91%. . m.p.136~138°C. 1 HNMR (CDCl 3 , 400 MHz) δ: 1.89-1.93(m, 2H, CH 2 ), 2.67-2.69 (m, 2H, CH 2 N), 2.77-2.78 (m, 4H, CH 2 N), 3.10-3.11(m, 4H, CH 2 N), 3.18-3.20 (m, 2H, CH 2 N), 3.33(s, 4H, CH 3 N), 3.42(s, 3H, CH 3 N), 3.89(s, 3H, CH 3 O), 4.80(s, H, CH=), 6.89-6.98(m, 3H, ArH), 7.04-7.08(m, H, ArH).
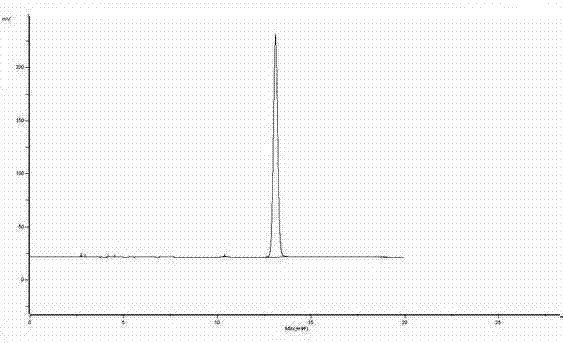
[0032] attached figure 1 The HPLC figure analysis result of urapidil standard substance is as follows:
[0033]
experiment example 1
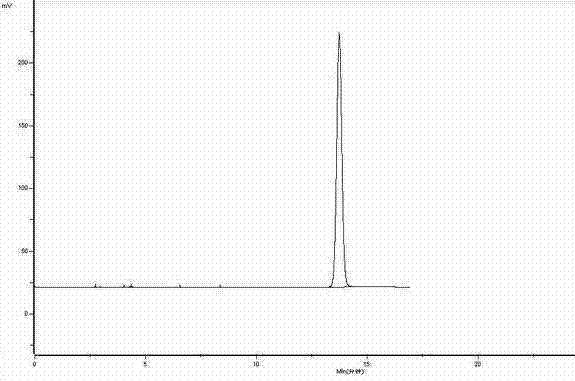
[0034] The HPLC figure analysis result of experimental example 1 gained urapidil is as follows:
[0035]
Embodiment 2
[0037] 1-(2-methoxyphenyl)piperazine hydrochloride (12.84g, 0.056mol) was added into K 2 CO 3 (11.9g, 0.086mol) and the mixture of 50ml water, be heated to 96 ℃, add 6-(3-chloropropyl)-1,3-dimethyluracil (10g, 0.043mol) in batches, then Add 0.4 g of cetyltrimethylammonium bromide, after the addition is complete, react for 4 hours, add water, stir and cool, a white substance precipitates, filter under reduced pressure, and dry at room temperature to obtain 13.82 g of a white powdery solid, yield 83.0% , with a purity of 99.83%. Melting point and spectral data are the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com