Application of a kind of sorbose reductase in biological preparation of ethyl (s)-4-chloro-3-hydroxybutyrate
A technology of ethyl hydroxybutyrate and ethyl chloroacetoacetate is applied in the field of sorbose reductase to achieve the effects of reducing production cost, high yield and high optical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
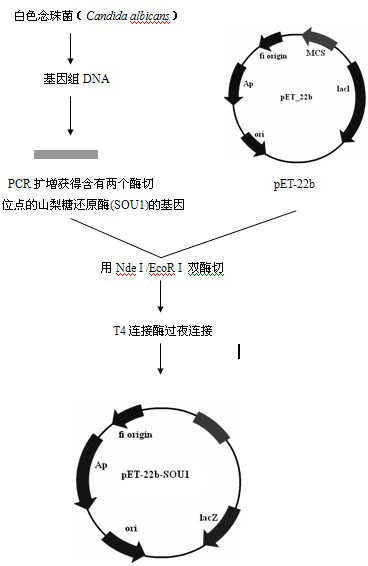
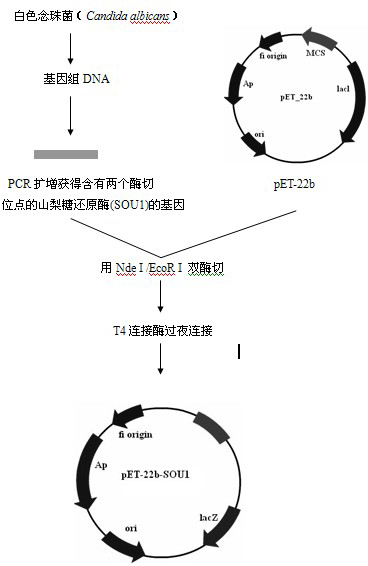
[0030] Step 1. Acquisition of sorbose reductase gene
[0031] Candida albicans Candida albicans (purchased from Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversiry Centre), medium YPD (g·L -1 ): Yeast extract 10g, peptone 20g, glucose 20g, add distilled water to 1L.
[0032] Candida albicans Candida albicans Inoculate in 5mL LYPD liquid medium and culture at 30°C until the logarithmic growth phase, and use the genomic DNA extraction kit (Beijing Tianwei Bioengineering Co., Ltd. Yeast Genome Extraction Kit) to extract the genome.
[0033] The primers used to construct the expression vectors are provided with enzyme cutting sites, and the primer sequences are as follows:
[0034] The upstream primer (SOU1-sense containing NdeI) is: 5'- GGAATTCCATATGATGAGTGAAGAAATTCATTTCA -3'
[0035] The downstream primer (SOU1-anti containing EcoRI) is: 5'- CCGGAATTCTTATGGACATGTATAACCCCCAT -3'
[0036] All primers were synthesized by Meiji Biotechnology Co., Ltd.
[0037] Gen...
Embodiment 2
[0059] As in Step 1 to Step 4 of Example 1, the precipitate was prepared for subsequent use. The above precipitate was washed twice with potassium phosphate buffer (100 mmol L-1, pH 6.5), and 0.5 g (wet weight) of E. coli sludge was weighed. Suspend in 25 mL of the total reaction solution (pH 6.5 potassium phosphate buffer to butyl acetate volume ratio of 1:1). Add mannitol 1300mmol / L, COBE 200g / L, 30°C, 180rpm, 16h. The yield of the product (S)-CHBE was 185g / L, the yield of the product was 92.5%, and the optical purity e.e% was 100%.
[0060] The detection method of product is with embodiment 1
Embodiment 3
[0062] As in Step 1 to Step 4 of Example 1, the precipitate was prepared for subsequent use. The above precipitate was washed twice with potassium phosphate buffer (100 mmol L-1, pH 6.5), and 0.5 g (wet weight) of E. coli sludge was weighed. Suspend in 25 mL of the total reaction solution (pH 6.5 potassium phosphate buffer to butyl acetate volume ratio of 1:1). Add xylitol 1300mmol / L, COBE 200g / L, 30°C, 180rpm, 16h. The yield of the product (S)-CHBE was 130 g / L, the yield of the product was 65%, and the optical purity e.e% was 100%.
[0063] The detection method of product is with embodiment 1.
[0064] Sequence List
[0065]
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com