Mycobacterium tuberculosis ag85ab chimeric gene vaccine, its preparation method and application
A technology of Mycobacterium tuberculosis and genetic vaccine, applied in the field of biomedicine, can solve the problems of unsatisfactory pathological damage, etc., and achieve the effect of strong anti-TB cellular immune response and better therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
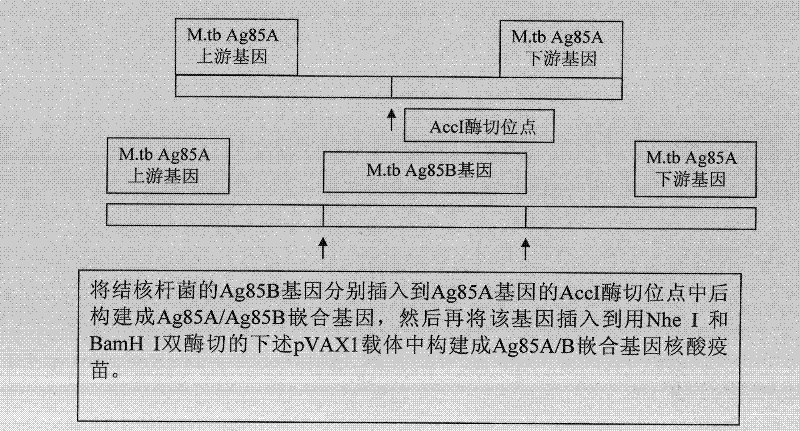
[0056] In a preferred embodiment, the preparation method of the chimeric Mycobacterium tuberculosis gene vaccine of the present invention comprises the following steps:
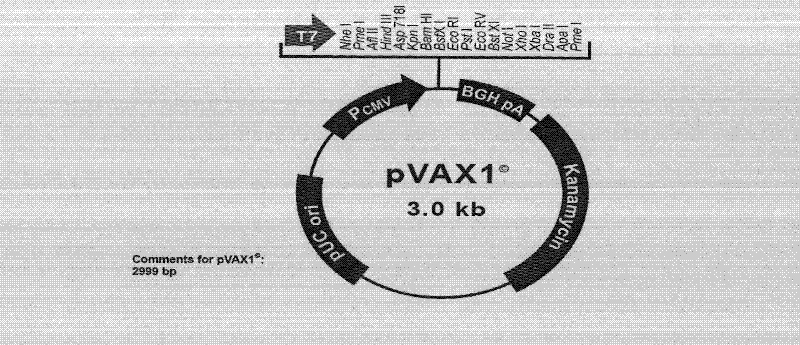
[0057] (1) Select the 245-250 KpnI restriction site or the 430-435 Acc I restriction site that can be inserted into the exogenous DNA fragment in the Ag85a gene, and use endonuclease Kpn I or endonuclease, respectively Acc I digests the previously constructed eukaryotic expression vector pVAX1 containing the Ag85a gene, linearizes it, and dephosphorylates it with alkaline phosphatase;
[0058] (2) Use a pair of recognition sequences with endonuclease Kpn I respectively GGTACC primers, or a pair of endonuclease Acc I recognition sequences GTCTAC The primers were used to amplify the DNA fragment encoding the amino acid sequence of Ag85b protein 125-282 by polymerase chain reaction;
[0059] (3) Connect the dephosphorylated linear Ag85a gene vector of step (1) and the DNA amplification fragment encoding the a...
Embodiment 1
[0071] Example 1: Preparation of nucleic acid vaccine containing Ag85ab chimeric gene pVAX1 plasmid (HG85abA plasmid)
[0072] Main experimental materials :
[0073] The pVAX 1 vector plasmid was purchased from Invitrogen
[0074] The pVAX1-85a containing the Mycobacterium tuberculosis Ag85a gene and the PET28A-85B plasmid containing the Mycobacterium tuberculosis Ag85b gene derived from the pVAX1 plasmid vector were prepared by Shanghai Haigui Biotechnology Co., Ltd. (Li Z, Song D, Zhang H, et al., Improved humorol immunity against Tuberculosis ESAT-6 antigen by chimeric DNA prime and protein boost strategy. DNA Cell Biol, 2006, 25(1):25-29).
[0075] Alkaline phosphatase and Taq DNA polymerase are products of NEB Company.
[0076]Restriction endonucleases BamH I, Hind III and dNTP Mix (mixture) are products of Takara Company. Restriction endonucleases Acc I, Kpn I and T4 DNA ligase are products of MBI Company.
[0077] AXYGEN plasmid extraction kit and DNA gel recovery...
Embodiment 2
[0124] Example 2: Preparation of nucleic acid vaccine containing Ag85ab chimeric gene PVAX1 plasmid (HG85abK plasmid)
[0125] Main experimental materials: Same as Example 1
[0126] Preparation
[0127] (1) Primer Design and Synthesis : Designed for PCR amplification of Ag85b gene (coding amino acids 125-282) fragment containing Kpn I restriction site The following upstream primer P3 and downstream primer P4 oligonucleotide sequences:
[0128] P3: 5'-CACATCACGATACCG TCGATGGCCGGCTCGTC-3' (SEQ ID NO: 6)
[0129] P4: 5'-CACATGCGAATACCG TAACGAACTCTGCAGGTC-3' (sequence 7)
[0130] The two sequences were sent to Invitrogen for synthesis.
[0131] (2) PCR amplification of the fragment of the Ag85b gene (encoding amino acids 125-282) : The method is the same as in Example 1, but the above-mentioned P3 / P4 primers are used.
[0132] (3) Preparation of dephosphorylated linear pVAX1-Ag85A plasmid : The method is the same as in Example 1, but Kpn I enzyme is used.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com