Transposon end compositions and methods for modifying nucleic acids
A technology of transposon and composition, applied in the field of composition and method of transposon end for modifying nucleic acid, can solve the problems of low efficiency, high cost, tediousness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
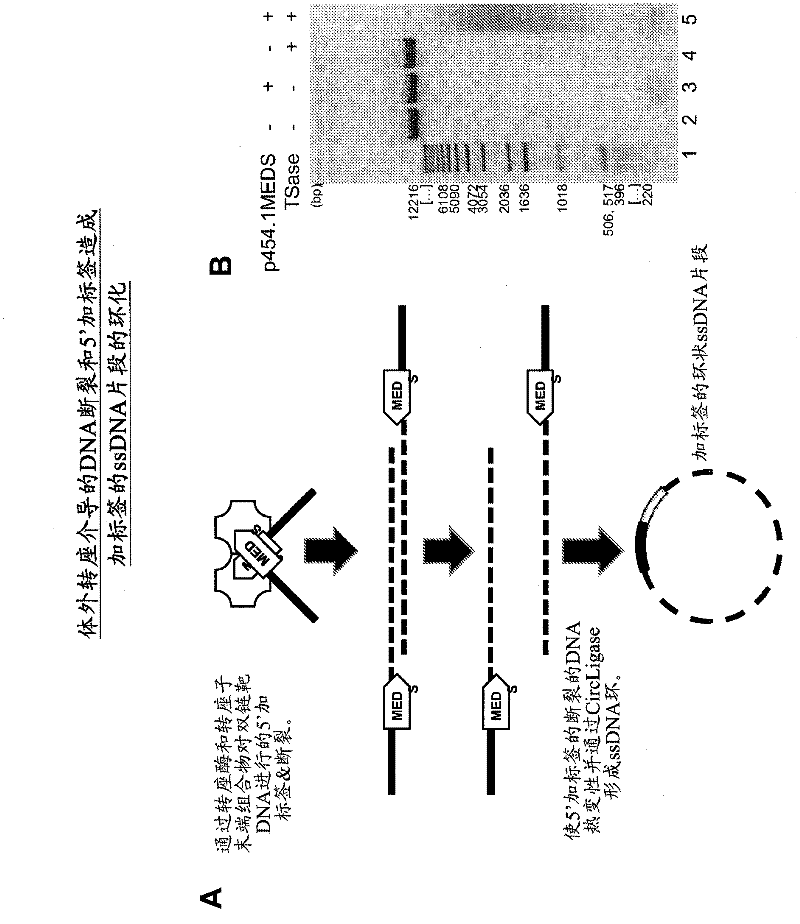
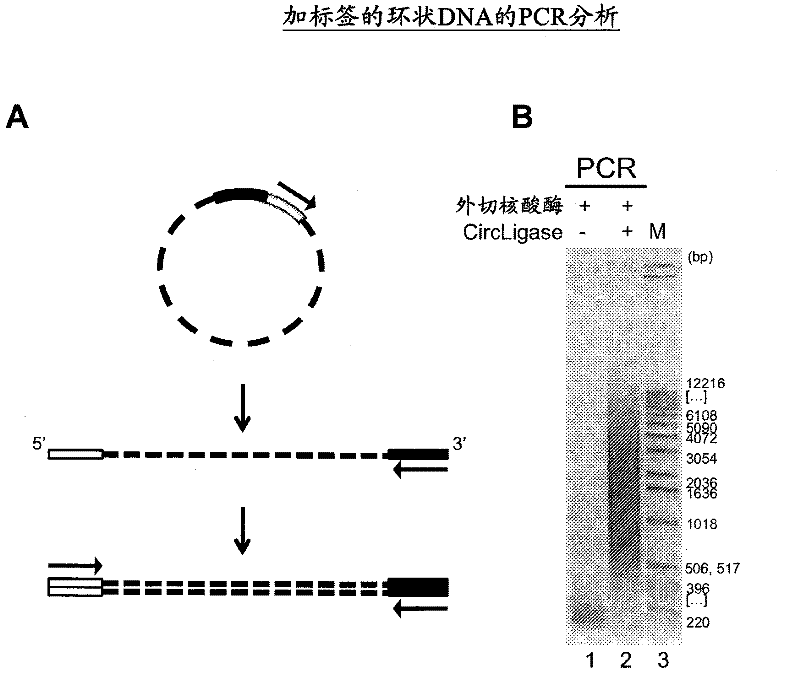
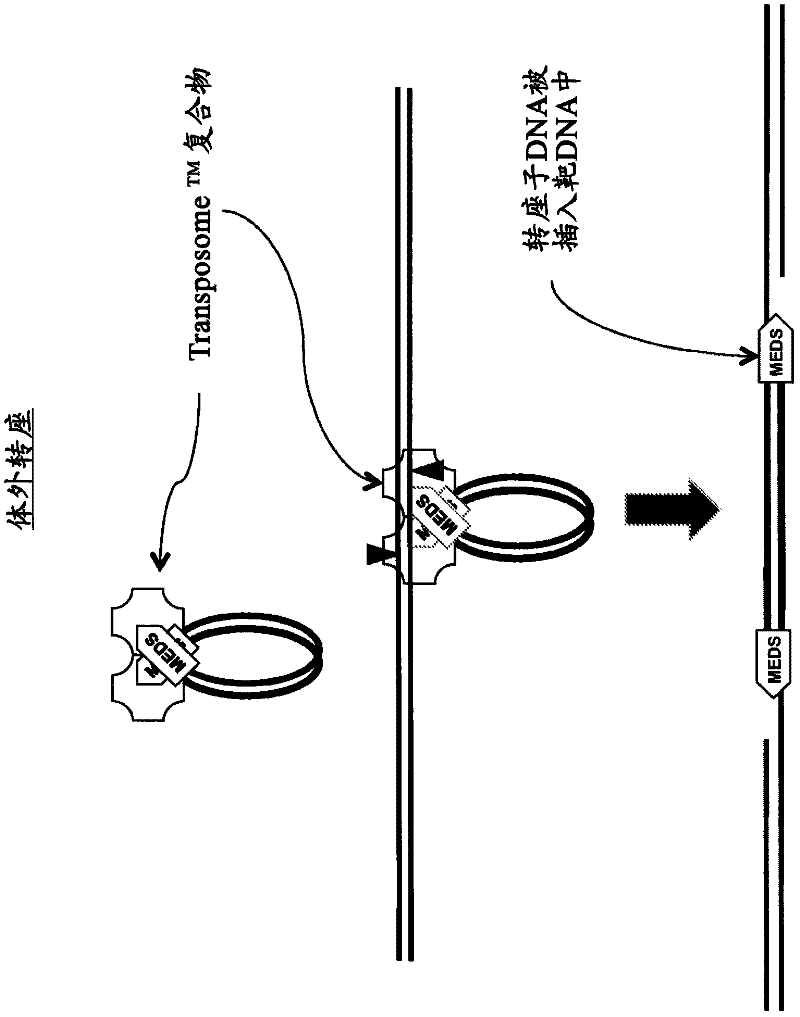
[0474] Using EZ-Tn5 TM Transposases and EZ-Tn5 TM In vitro transposition-mediated DNA fragmentation and 5' tagging of transposon ends
[0475] The following reaction mixtures were assembled:
[0476]
[0477] * In some embodiments, two different pMEDS transposon ends ( Figure 4 ).
[0478] After mixing, the reaction was incubated at 37°C for 1 hour. The reaction was stopped with 10 microliters of stop solution (15% sucrose, 66 mM EDTA, 20 mM TRIS, pH 8.0, 0.1% SDS, 0.9% Orange G [Sigma O-7252], and 100 micrograms per milliliter of proteinase K), mixed and Heat at 50°C for 10 minutes.
[0479] DNA was analyzed by 1% agarose gel electrophoresis in TAE buffer. DNA was separated into size grades using LMP agarose. Gels were stained with SYBR Gold and DNA was visualized with non-UV light. The gel slices of LMP were incubated at 70°C for 5 minutes to liquefy the gel. After 5 minutes at 37°C, add one percent volume of Gelase TM Agarose Digestion Solution (EPICENTRE Bio...
Embodiment 2
[0482] Use different EZ-Tn5 TM Size range of transposition products of 5'-tagged DNA fragments at Tn5 transposase concentration.
[0483] Add Tn5 highly active EZ-Tn5 at a concentration of 90 units per microliter TM Transposase (EPICENTRE) was diluted to final concentrations of 45, 22.5, 11.3 and 9 units per microliter. Combine two microliters of each concentration of enzyme with 1 microgram of bacteriophage T7 D111 target DNA (with a size of approximately 39 Kbp) and 1 micromolar of pMEDS transposon ends in TA buffer in 50 microliters of final reaction mixture at 37 °C Incubate in volume for 1 hour.
[0484] Reactions were stopped with 10 microliters of stop solution containing 15% sucrose, 66 mM EDTA, 20 mM Tris / HCl pH 8.0, 0.1% SDS, 0.9% Orange G and 100 micrograms per milliliter proteinase K. After mixing and incubating for 10 minutes at 50°C, 10 microliter aliquots were electrophoresed on a 1% agarose gel in TAE buffer at 100 volts for 1 hour. Gels were stained with ...
Embodiment 3
[0487] Size range of transposition products of 5′-tagged DNA fragments using different concentrations of pMEDS transposon ends.
[0488] use T 10 E 1 Buffer 25 micromolar stocks of pMEDS transposon ends were serially diluted 2-fold, 4-fold and 8-fold. Then, 2 μl of each transposon-end dilution and no transposon-end buffer control in a buffer containing 1×TA buffer, 1 μg of phage T7 D111 target DNA, and 0.4 units / μl of high temperature at 37°C. Incubate 50 µl reactions of active Tn5 transposase for 1 hr.
[0489] Reactions were terminated as described in Example 2 and samples were analyzed by 1% agarose gel electrophoresis.
[0490] A 4-fold dilution of the 25 uM stock to give a final concentration of 0.25 micromolar pMEDS transposon ends in the reaction mixture produced good fragmentation of the target DNA and was probably the most efficient in terms of use of pMEDS transposon ends. At this concentration, most of the phage T7 D111 target DNA was fragmented into DNA that mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com