Determination method of practical solubility of calcium sulfate for papermaking filler
A measurement method and technology of paper-making fillers, which are applied in the direction of weighing by removing certain components, can solve the problems that the loss of calcium sulfate fillers cannot be objectively reflected, and the actual retention rate of the fillers in the measurement results cannot be mutually verified, so as to achieve the operation steps simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Calcium sulfate test sample is natural mechanically ground hydrous gypsum. The determination steps are as follows:
[0026] (1) Pretreatment: take about 50g of calcium sulfate sample, place it in a controllable drying oven at (105±2)°C and dry it to constant weight, and set aside;
[0027] (2) Sampling: take by weighing 2.0012g of the sample gained in step (1) with an analytical balance of one ten-thousandth;
[0028] (3) Dispersion: Place the sample obtained in step 2 in a 500ml glass beaker, add 200ml of deionized water to disperse, and the resulting dispersion concentration is 0.99%;
[0029] (4) Heating and stirring: heat the dispersion obtained in step (3) in a water bath, keep the heating temperature of the dispersion at 40°C, and continue to stir gently for 60 minutes;
[0030] (5) Suction filtration: place the filter paper with constant weight (0.9312g) in the Buchner funnel, carry out suction filtration to the dispersion obtained in step (4), and use 150ml de...
Embodiment 2
[0035] In a smelter, the industrial waste slag of non-ferrous metals purified by the solvent method reacts with calcium oxide to produce fibrous synthetic calcium sulfate whiskers. The whiteness of the products is >90%; the average aspect ratio of the whiskers is 38:1; it is burned at 900°C Weight loss of 16.7%; filler for cultural printing paper.
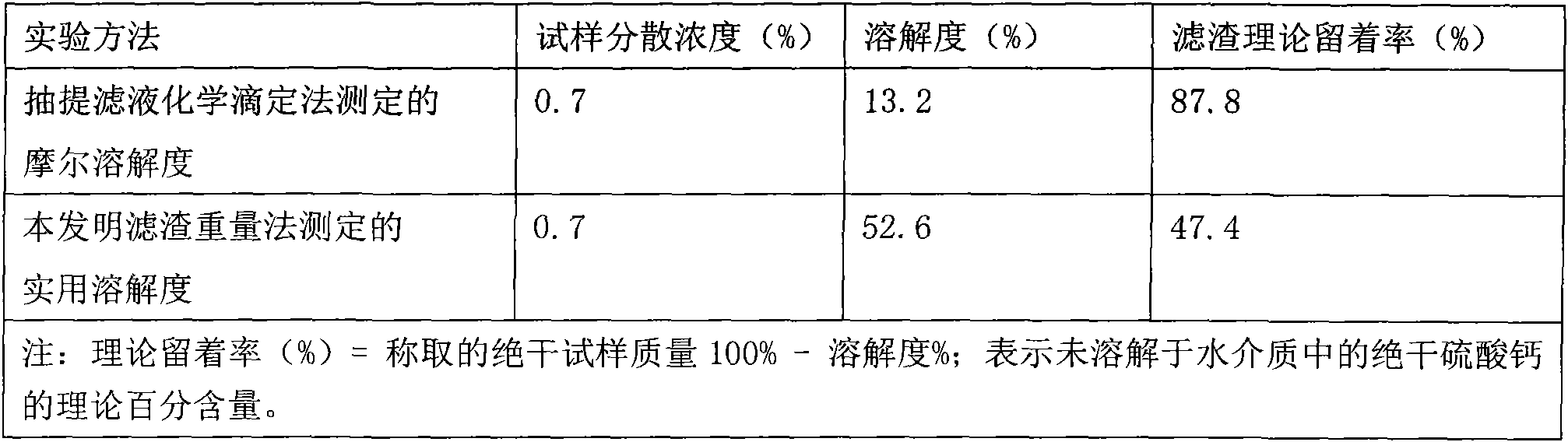
[0036] Take 3.15 g of the pretreated dry synthetic calcium sulfate whisker sample, and disperse it for 60 min at a concentration of 0.7% and a temperature of 50°C. Adopt two kinds of solubility experimental methods to measure the solubility index of synthetic calcium sulfate whisker and list Table 1.
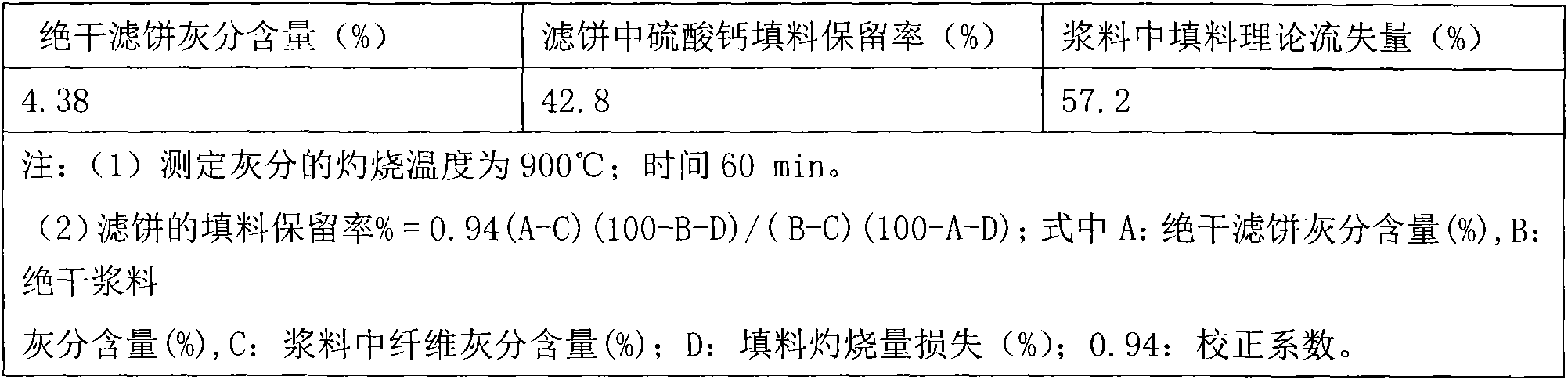
[0037] Calcium sulphate whiskers are used as paper fillers, and the amount added to the pulp is 25%; the concentration of the slurry is 0.5%, the ash content of the slurry is 5.58%, and the ash content of the plant fiber in the slurry is 3.37%. The filler retention experiment was carried out in the DFS-03 papermaking slurry dynamic ...
Embodiment 3
[0044] A paper mill uses the industrial waste residue produced by desulfurization in a thermal power plant to react with sulfuric acid to produce synthetic calcium sulfate, which is used as a filler for cultural printing paper.
[0045] One, adopt the inventive method to measure the practical solubility of synthetic calcium sulfate, and assay step is as follows:
[0046] (1) Pretreatment: Take 50 g of synthetic calcium sulfate whiskers and dry them to absolute dryness for later use, at a drying temperature of (105±2)°C;
[0047] (2) Sampling: take by weighing 2.0004 g of the sample gained in step (1) with an analytical balance with a sensitivity of one ten thousandth;
[0048] (3) dispersion: the sample of step (2) gained is placed in the glass beaker of 500ml, adds the deionized water dispersion of 300ml, and gained dispersion concentration is 0.66%;
[0049] (4) Heating and stirring: heat the dispersion obtained in step (3) in a water bath and continue to stir gently for 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com