A kind of l-type zeolite/polymer luminescent material and preparation method thereof
A technology of luminescent materials and high polymers, applied in the field of high polymers, can solve problems such as strong light scattering, achieve the effects of suppressing leakage, enhancing stability, and improving physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
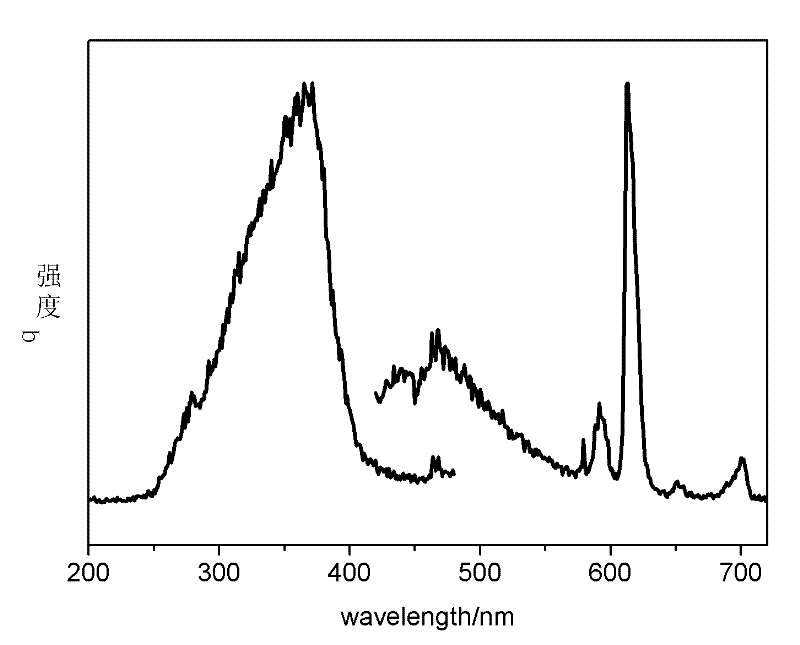
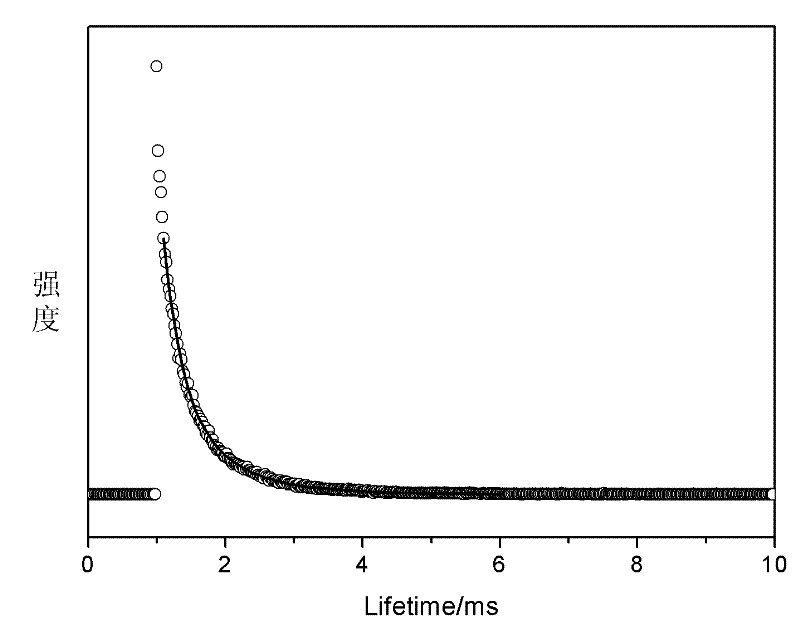
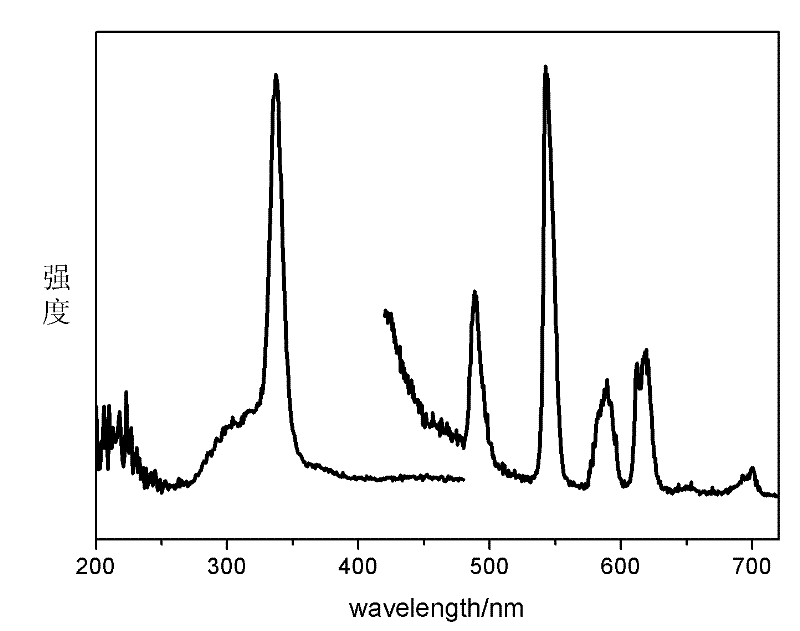
[0038] In the first step, take 100 mg of nano-zeolite crystals and add to 2.5 mL of EuCl with a concentration of 0.1 mol / L 3 solution, stirred at 80°C for 24h, centrifuged, discarded the supernatant, washed the precipitate with deionized water and dried in a drying oven at 80°C for 3h to obtain doped Eu 3+ L-type zeolite, take the doped Eu 3+Mix 100 mg of L-type zeolite with organic ligands 2-thiophene trifluoroformyl acetone (TTA) and 4 mg of o-phenanthroline (phen). The rotary vane vacuum pump was used to evacuate for 2 hours, and then heated at a constant temperature of 120°C for 48 hours, then the product was taken out, washed with dichloromethane four times to remove organic matter that was not mixed into the zeolite pores, and then placed in a vacuum at 60°C. Dry in a vacuum oven at 133 Pa for 12 hours to prepare nano-zeolite NZ-Eu-TTA-phen (98.3 mg) loaded with rare earth Eu-organic complexes. The product obtained in this step can be observed under ultraviolet light to...
Embodiment 2
[0045] with TbCl 3 and EuCl 3 The mixed solution replaces the EuCl of embodiment 1 3 solution. 2,2'-bipyridine (Bipy) replaces 2-thiophene trifluoroformyl acetone (TTA), o-phenanthroline (phen), and the specific steps are as follows:
[0046] 1. Take 100mg of nano zeolite crystals, according to Eu 3+ :Tb 3+ The molar ratio is 9:1, that is, 2.25mL of 0.1mol / L EuCl 3 solution with 0.25mL 0.1mol / L TbCl 3 The solution is mixed well.
[0047] 2. Ion-exchange the mixed liquid with nano-zeolite, mix it with Bipy after drying, grind it, put it into a long-necked bottle, evacuate it with a rotary vane vacuum pump with a vacuum degree of 1 Pa for 2 hours at 80°C, and then heat it at a constant temperature at 100°C After 48 hours, the product was taken out, washed four times with dichloromethane to remove organic matter not doped into the pores of the zeolite, and then placed in a vacuum oven at 60°C and a vacuum of 133Pa for 12 hours to obtain nanopores. Nano zeolite NZ-9Eu-1Tb-...
Embodiment 3
[0049] Change the molar ratio of the first step in Example 2 to 8:2, that is, 2 mL of 0.1 mol / L EuCl 3 solution with 0.5mL 0.1mol / L TbCl 3 The solution is mixed well. All the other processes are the same as in Example 2. Finally, a transparent zeolite / polymer luminescent material is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com