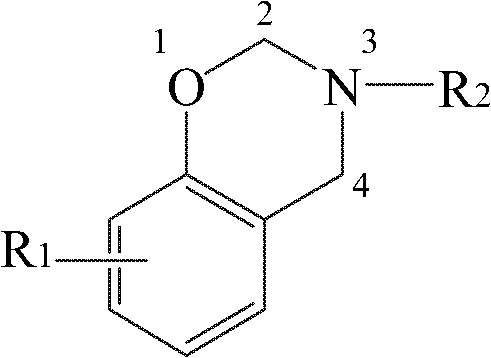
Dibenzoxazine containing oxazole ring and preparation method thereof
A technology of benzoxazine and fenoxazole rings, which is applied in the field of bisbenzoxazine and its preparation, can solve the problems of high curing temperature, inconvenient processing, and unsatisfactory conditions, and achieve low equipment requirements, simple preparation process, thermal good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A kind of preparation method of the dibenzoxazine containing oxazole ring, concrete steps are,
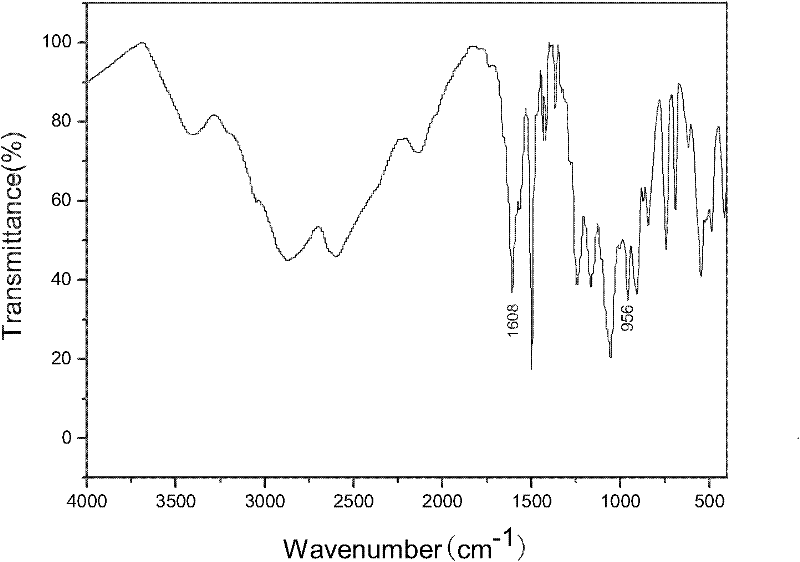
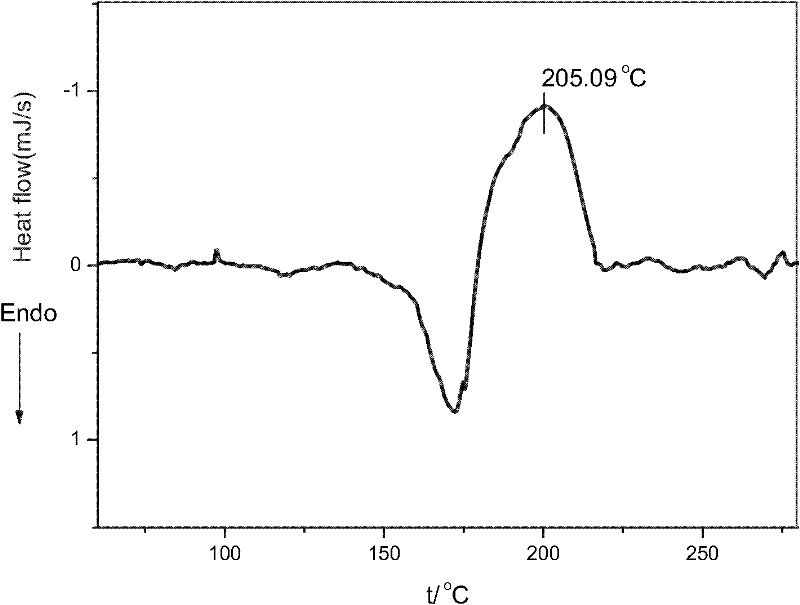
[0042] (1) 5.00g of 4,6-diaminoresorcinol dihydrochloride (abbreviated as DAR), 75gPPA and 6.48g p-hydroxybenzoic acid are added to the reaction flask equipped with stirrer, thermometer and condenser tube , and heated to 60°C for stirring. Subsequently, the reaction device was evacuated with a vacuum pump, and then filled with nitrogen, and this was repeated three times. Then, under the condition of feeding nitrogen for protection, the temperature of the system was raised to 90°C at a rate of 10°C / hour, the nitrogen flow was stopped, and the foam in the reaction bottle was removed by vacuuming, and repeated several times until the foam was stable, and then respectively React at 90°C for 6 hours, 100°C for 6 hours, 130°C for 8 hours, 150°C for 8 hours, and 180°C for 8 hours. Cool to below 80°C, add 300mL of distilled water into it, stir, boil for 10 minutes, then cool natur...
Embodiment 2
[0051] The DAR in Example 1 is replaced by 6CDAR, the amount of reactants is changed accordingly, and other operating steps are the same as those in Example 1.
[0052] The specific chemical structure of 6CDAR is:
[0053]
[0054] In the first step reaction, the amount of reactant is changed to: 6CDAR is 5.00g, PPA is 75gPPA, p-hydroxybenzoic acid 4.16g.
[0055] In the second step reaction, the amount of the reactant was changed to: 1.00 g of diphenol obtained from the previous step reaction, 0.26 g of paraformaldehyde, and 0.40 g of aniline.
[0056] The structural formula of gained oxazine monomer is:
[0057]
[0058] After ring-opening polymerization of the oxazine monomer obtained in this example, the dielectric constant of the polymer is 2.0, the 5% weight loss temperature of the polymer is 483° C., and the residual carbon rate at 800° C. is 57%.
Embodiment 3
[0060] In Example 1, DAR in Example 1 is replaced by 6FDAR, aniline is replaced by m-aminophenylacetylene, and the amount of reactants is changed accordingly. Other operating steps are the same as those in Example 1.
[0061] The specific chemical structure of 6FDAR is:
[0062]
[0063] In the first step reaction, the amount of reactant is changed to: 6FDAR is 5.00g, PPA is 75gPPA, p-hydroxybenzoic acid 3.14g.
[0064] In the second step reaction, the amount of the reactant was changed to: 1.00 g of diphenol obtained from the previous step reaction, 0.21 g of paraformaldehyde, and 0.41 g of m-aminophenylacetylene.
[0065] The structural formula of the obtained oxazine monomer is:
[0066]
[0067] After ring-opening polymerization of the oxazine monomer obtained in this example, the dielectric constant of the polymer is 1.7, the 5% weight loss temperature of the polymer is 550° C., and the residual carbon rate at 800° C. is 61%.
[0068] The advantage of the present ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal resistance | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com