Preparation method and application of eclipta extract
A Eclipta chinensis extract and Eclipta chinensis technology are applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, can solve problems such as the Eclipta chinensis extract has not been disclosed, and achieve environmental pollution-free, reduced The effect of purification costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The steps of the preparation method of Eclipta chinensis extract are as follows:
[0017] 1) Select the Eclipta chinensis plant, soak it in ethanol / water with a volume percentage concentration of 5-95% for 0.5 hours, extract 1 hour with ethanol / water that is 20 times the weight of the medicinal material Eclipta chinensis, and ethanol / water is the ink Extract 16 times the weight of the Eclipta medicinal material for 0.5h, combine the extract, concentrate the extract to 0.25-1.0g crude drug / mL, let it stand for 6h, and filter to obtain the Eclipta extract;
[0018] 2) Eclipta extract is adsorbed by pretreated macroporous resin, the weight ratio of Eclipta extract to macroporous resin is 1:1~1:5, and the sample flow rate is 1~3 BV / h. The ratio of diameter to height is 1:4~1:7. After adsorption, wash with water for 3~6 BV, the elution flow rate is 2~4BV / h, and discard the water eluent; then use 20~90% ethanol with volume percentage concentration / water elution of 3-6 BV, t...
Embodiment 1
[0027] Embodiment 1: the preparation of Eclipta chinensis extract
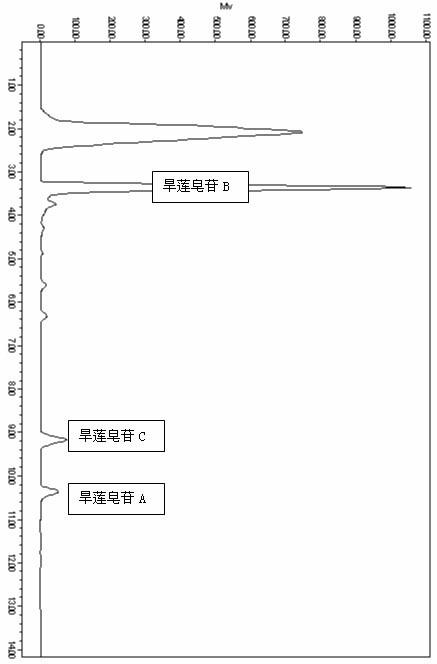
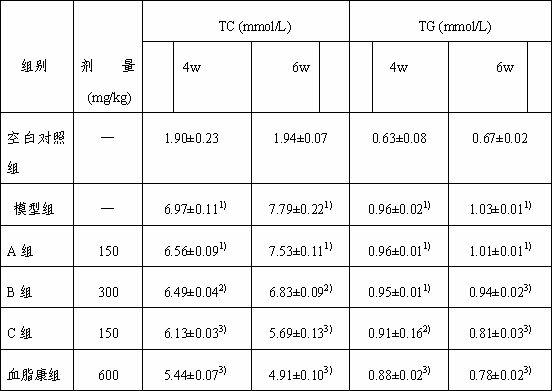
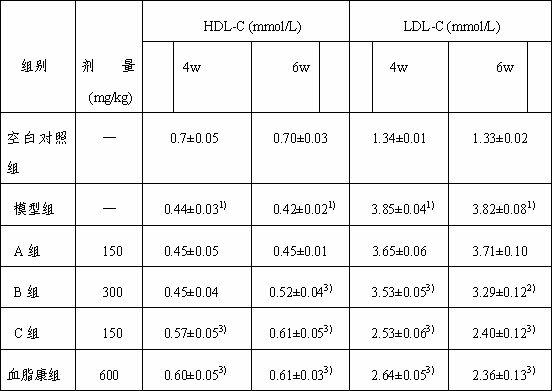
[0028] Eclipta medicinal material 10kg, soaked in ethanol / water with a volume percentage concentration of 5% for 0.5 hours, the ethanol / water is 20 times the weight of the medicinal material Eclipta 1h and the ethanol / water is the extraction of 16 times the weight of the medicinal material Eclipta 0.5 h, combine the extracts, concentrate the extracts to 0.25 g crude drug / mL, let stand for 6h, and filter to obtain the extract of Eclipta chinensis; The weight ratio of the resin is 1:1, the sample loading flow rate is 1 BV / h, and the diameter-to-height ratio of the resin column is 1:4. The volume percentage concentration was 20% ethanol / water to elute 3 BV, the flow rate was 2BV / h, and the volume percentage concentration was 20% ethanol / water eluate, concentrated and dried to obtain the Eclipta officinalis extract. The mass percent contents of Eclipta saponin B, A, and C in the Eclipta japonica extract were me...
Embodiment 2
[0029] Embodiment 2: the preparation of Eclipta chinensis extract
[0030]Eclipta medicinal material 10kg, soaked in ethanol / water with a volume percentage concentration of 95% for 0.5 hours, the ethanol / water is 20 times the weight of the medicinal material Eclipta 1h and the ethanol / water is the extraction of 16 times the weight of the medicinal material Eclipta 0.5 h, combined the extracts, concentrated the extracts into 1.0 g crude drug / mL, left standstill for 6h, and filtered to obtain the Eclipta extract; the Eclipta extract was passed through the LX60 macroporous resin, and the Eclipta extract and the macroporous resin were separated The weight ratio is 1:5, the sample loading flow rate is 3BV / h, and the diameter-to-height ratio of the resin column is 1:7. After adsorption, wash 6BV with water first, and the elution flow rate is 4BV / h. The concentration is 90% ethanol / water to elute 6 BV, the flow rate is 4 BV / h, the eluate with a volume percentage concentration of 90...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com