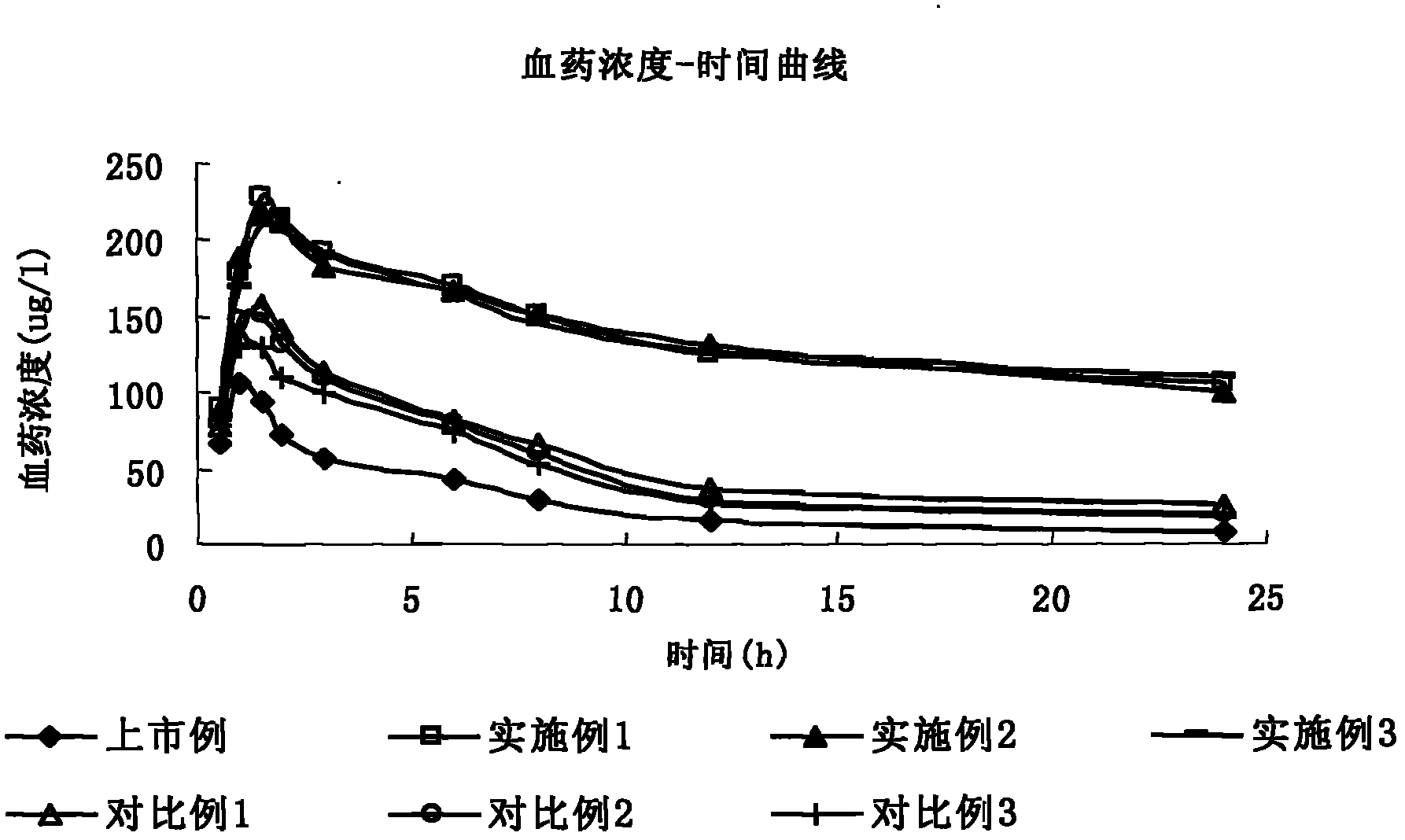
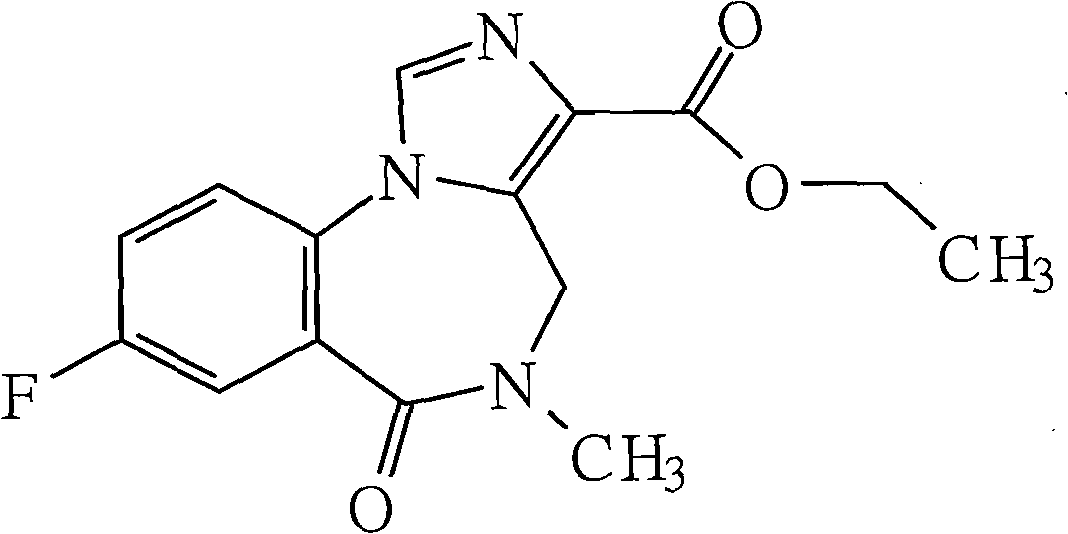
Flumazenil liposome injection
A technology of flumazenil and mazenil, which is applied in the directions of liposome delivery, organic active ingredients, nervous system diseases, etc., can solve the problems of poor automatic dispersion uniformity, increase the manufacturing cost, and affect the use effect, etc. The effect of improving quality, improving solubility, and improving efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] On the other hand, the present invention also provides a preparation method of flumazenil liposome injection, specifically comprising the following preparation steps:
[0076] (1) Cholesterol, phosphatidylethanolamine, soybean sterol, and Tween 80 are dissolved in an appropriate amount of buffered saline solution to make blank liposomes;
[0077] (2) The blank liposomes prepared above were sterilized by circulating steam, and then ultrasonically treated twice, each time for 15 minutes;
[0078] (3) under sterile conditions, add flumazenil to the liposome in molten state, add trehalose and PVP under constant stirring;
[0079] (4) Filtrate with a 0.45um microporous membrane, freeze quickly, then return to room temperature, and fill to obtain the flumazenil liposome injection.
[0080] The above-mentioned preparation method, wherein said buffered saline solution is selected from one of phosphate buffered saline, citrate buffered solution, carbonate buffered solution, bor...
Embodiment 1
[0088] The preparation of embodiment 1 flumazenil liposome injection
[0089] The ingredients used and their weights are as follows:
[0090]
[0091] Adopt preparation process to prepare flumazenil liposome injection:
[0092] (1) 120g cholesterol, 150g phosphatidylethanolamine, 30g soy sterol and 10g Tween 80 are dissolved in 3000mlpH in the phosphate buffered saline solution that is 7.2, make blank liposome;
[0093] (2) The blank liposomes prepared above were sterilized by circulating steam, and then ultrasonically treated twice, each time for 15 minutes;
[0094] (3) Under sterile conditions, add 1 g of flumazenil to the liposome in a molten state at 60° C., and add 100 g of trehalose and 150 g of PVP under constant stirring;
[0095] (4) Filtrate with a 0.45um microporous membrane, freeze quickly at -80°C, then return to room temperature, and fill to obtain the flumazenil liposome injection.
Embodiment 2
[0096] The preparation of embodiment 2 flumazenil liposome injection
[0097] The ingredients used and their weights are as follows:
[0098]
[0099]
[0100] Adopt preparation process to prepare flumazenil liposome injection:
[0101] (1) 75g cholesterol, 100g phosphatidylethanolamine, 25g soybean sterol and 20g Tween 80 are dissolved in the phosphate-buffered saline solution of 7.2 in the pH of 3000ml, make blank liposome;
[0102](2) The blank liposomes prepared above were sterilized by circulating steam, and then ultrasonically treated twice, each time for 15 minutes;
[0103] (3) Under sterile conditions, add 0.5 g of flumazenil to the liposome in a molten state at 60° C., and add 30 g of trehalose and 40 g of PVP under constant stirring;
[0104] (4) Filtrate with a 0.45um microporous membrane, freeze quickly at -80°C, then return to room temperature, and fill to obtain the flumazenil liposome injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com