RANKL-TNF (Receptor Activator of Nuclear Factor Kappa-B Ligand-Tumour Necrosis Factor) sample region fusion protein and preparation method and application thereof
A kind of fusion protein, protein technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
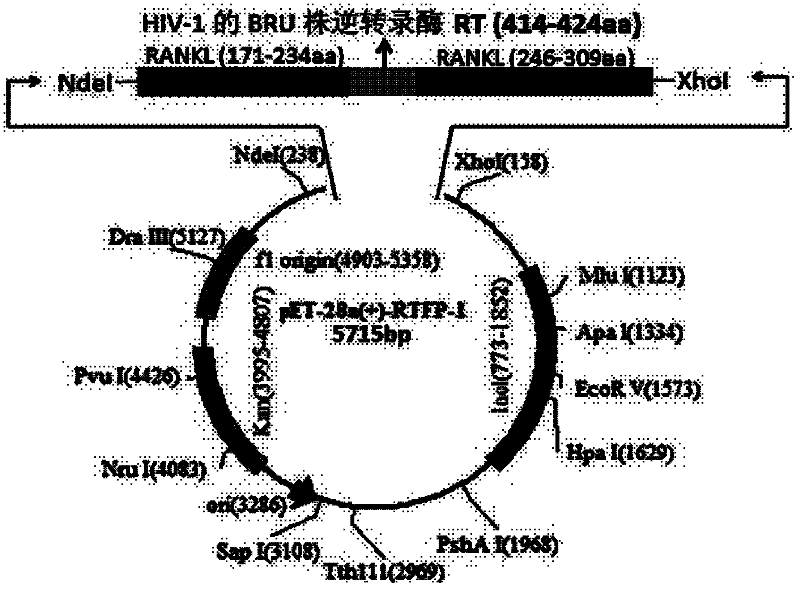
[0040] Example 1 Construction of RANKL-TNF-like region fusion protein prokaryotic expression vector
[0041] Primers were designed according to the gene sequence of the RANKL-TNF-like region recombinant fragment (RANKL P171-234, P246-309, HIV-1 BRU strain reverse transcriptase RT (414-424aa)) and the characteristics of the pET-28a(+) expression vector ( See the table below), using the full-length cDNA of RANKL as a template (Gen Bank NO: BC117286, 1097bp; pDONR223, 5004bp), retaining the His tag of pET-28a, and connecting the three gene fragments by recombinant PCR method, wherein The gene sequence of the BRU strain reverse transcriptase RT (414-424aa) of HIV-1 is inserted in the middle of the two RANKL-TNF sample region gene fragments, and the expression vector obtained by constructing is pET-28a(+)-RTFP-1; The gene sequence of the BRU strain reverse transcriptase RT (414-424aa) of HIV-1 is inserted at the end of the two RANKL-TNF sample region gene fragments, and the express...
Embodiment 2
[0045] Example 2 Induced expression of RANKL-TNF-like region fusion protein in Escherichia coli
[0046] Transform Escherichia coli BL21 (DE3) competent cells with the correct sequenced recombinant cloned plasmid, pick a single colony and inoculate it in 10 mL LB medium (containing 30 mg / L kanamycin), and culture overnight at 37°C with shaking at 250 rpm. The next day, take 30 mL of the overnight culture and transfer it to 1 L of LB medium (containing 30 mg / L of kanamycin), and culture it to OD at 37°C and 250 rpm. 600 to 0.5, add IPTG to make the final concentration 0.1mM, continue shaking culture at 37°C, 250rpm for 4.5h, and collect the bacteria by centrifugation. SDS-PAGE and Western-blot analysis show that a large amount of fusion protein can be obtained after being induced by 0.4mMIPTG, see image 3 , 4.
Embodiment 3
[0047] Example 3 Purification and renaturation of RANKL-TNF-like region fusion protein
[0048] The positive clones were cultured at 0.4mM IPTG at 37°C for 4.5h, then centrifuged at 6000rpm at 4°C for 15min to collect the bacteria, washed with pre-cooled PBS for 6 times, and ultrasonically resuspended for 10min (over 4s and stop for 6s, power: 40%). Add 10 times the volume of bacterial cell protein lysate per gram of wet bacteria to resuspend, add lysozyme to 1mg / mL, and add protease inhibitor PMSF to 1mM at the same time, place on ice for 30min, then ultrasonically lyse the bacteria in an ice bath for 10min, The precipitate was collected by centrifugation at 12,000 rpm for 10 min. Wash once with TE buffer and 1% trixton-100 respectively, dissolve the precipitate in PBS and resuspend the bacteria by ultrasonic (5mL / g wet bacteria), at the same time slowly add an equal volume of 8M urea solution dropwise, magnetic stirrer Stir for 30 min, centrifuge and wash the precipitate o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com