Inactivation method for snake venom and inactivated snake venom prepared thereby
A snake venom and inactivation technology, applied in the field of snake venom inactivation and toxicity inactivation, can solve the problems of animal immune intolerance, conformational change of snake venom antigen, low neutralizing antibody titer, etc. Reduce antigen dosage and produce rapid results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Iodoacetamide method to inactivate viper venom:
[0030] The viper venom was purchased from Guangzhou Institute of Snake Venom, which is a freeze-dried toxin. Accurately weigh 100 mg of lyophilized toxin and dissolve it in 1000 ml of 50mM pH7.8 Tris buffer. After the snake venom is dissolved, centrifuge at 4000 rpm for 30 minutes to remove precipitated particulate matter. The supernatant is slowly poured into 3000 ml of iodoacetamide solution (50mM Tris , 1.33mM EDTA, 2.67M NaCl, 2.67M urea, 266.7mM iodoacetamide (pH 8.0), mix well and place in a 37°C water bath for 3 hours to avoid light. After the reaction, ultrafiltration was performed with a Millipore plate ultrafilter with a molecular weight cut-off value of 3000d. At the same time, the buffer was changed to pH7.0, 50mM Tris, 0.15M sodium chloride buffer, and the final protein concentration was concentrated to 1mg / ml, and stored at low temperature for later use.
Embodiment 2
[0031] Example 2. Inactivation of viper venom by formaldehyde method:
[0032] Take 100 mg of the above freeze-dried snake venom and add 5 ml of phosphate buffer solution to prepare a solution containing 20 mg of snake venom per ml. Add 50 microliters of formalin to the snake venom solution, shake well, and treat at room temperature for 7 days for inactivation ; The obtained inactivated attenuated snake venom is used as an immune antigen.
Embodiment 3
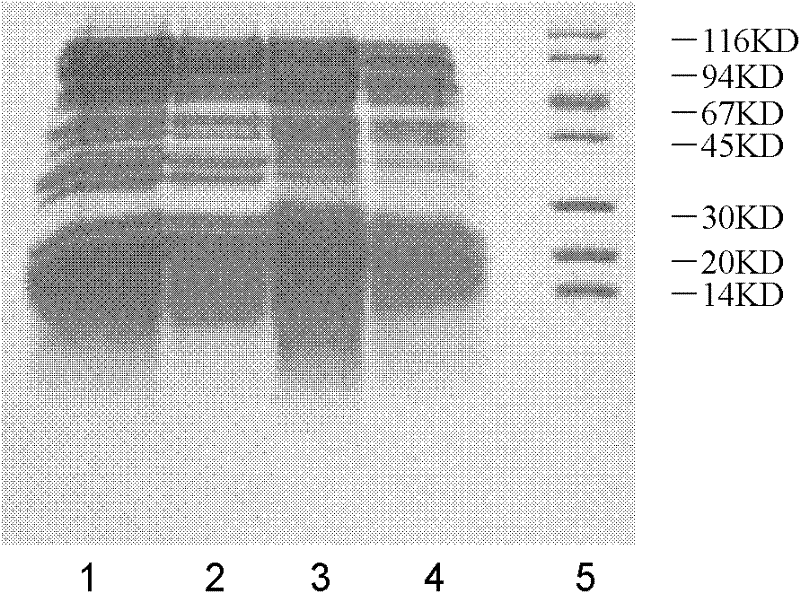
[0033] Example 3. SDS-PAGE comparison of electrophoretic characteristics of inactivated snake venom by two different methods
[0034] Comparison of inactivated snake venom and formaldehyde inactivated SDS-PAGE: After the snake venom was treated with iodoacetamide and dialyzed, it was separated on SDS-PAGE with a voltage of 150V (12.5% separation gel, 4 %Laminated glue)( figure 1 ), the formaldehyde-inactivated snake venom was separated on SDS-PAGE with a voltage of 150V (12.5% separation gel, 4% layering gel) ( figure 2 ), the gel was stained with Coomassie Brilliant Blue R-250 after electrophoretic separation. It can be seen from SDS-PAGE that the formaldehyde-inactivated snake venom forms a high-molecular polymer. figure 1 Compared with the snake venom before and after treatment with iodoacetamide, its molecular weight is significantly increased and dispersed, indicating that the proteins are cross-linked with each other, even in the presence of SDS and reducing agent, hea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com