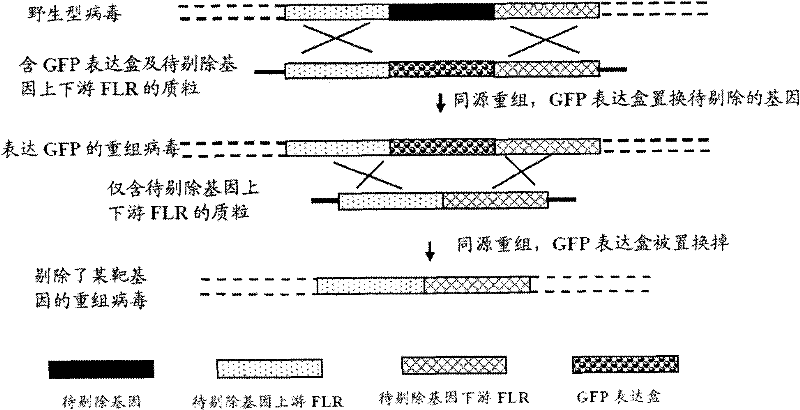
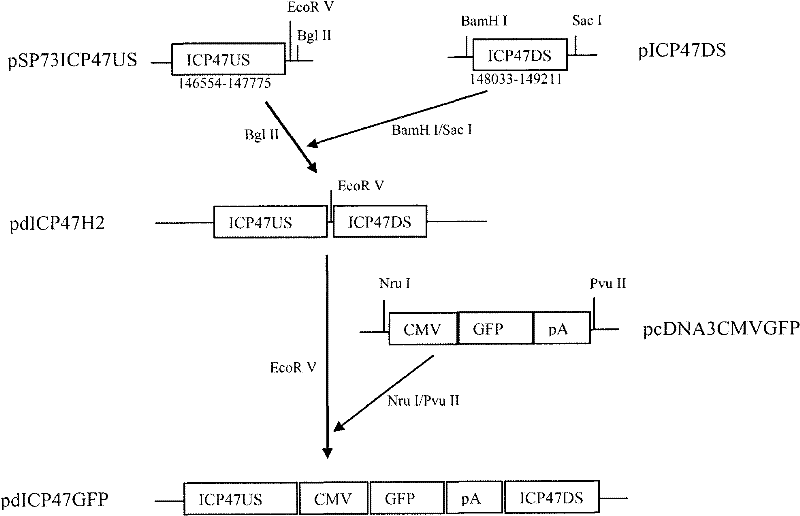
Recombined II-type herpes simplex virus, preparation method and application and tumour diagnostic reagent kit
A herpes simplex virus and virus technology, applied in the field of recombinant herpes simplex virus type Ⅱ, its preparation and application, and tumor diagnostic kits, can solve the problems of limited tumor types, low detection rate of micrometastases, and poor detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention also provides a method for preparing recombinant herpes simplex virus type II, wherein the method comprises deleting the ICP34.5 gene from the wild-type herpes simplex virus type II strain HG52 to construct recombinant herpes simplex virus type II HG52d34.5- Fluorescent protein steps:
[0036] A. Extract the full-length viral DNA of the wild type II herpes simplex virus HG52 strain;
[0037] B. Construction of the plasmid pH2dI34.5 containing the upstream flanking region sequence and the downstream flanking region sequence of the ICP34.5 gene:
[0038] B1. Use the primers shown in Table 1, and use the full-length viral DNA obtained in step A as a template to amplify the upstream flanking region sequence and the downstream flanking region sequence of the ICP34.5 gene by PCR;
[0039] Table 1
[0040]
[0041] B2. Insert the upstream flanking region sequence of the PCR product amplified in step B1 into the PvuII / XbaI site of the pSP72 plasmid to...
Embodiment 1
[0102] This example is used to illustrate the recombinant type II herpes simplex virus of the present invention.
[0103] Delete the ICP47 gene from the wild-type HSV-2 (HG52) genome to construct HG52dICP47 .
[0104] (1) DNA of purified HG52 wild-type virus
[0105] BHK cells were used to grow wild-type HG52 virus, and DNAzol TM Genomic DNA Isolation Kit (Helena Biosciences Cat. No. DN127200) was used to purify viral DNA. BHK cells were grown in a 175 cm square culture flask, and the culture medium was DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin. The culture conditions were 37°C, 5% carbon dioxide. When the cells were grown to 90% saturation, the virus was inoculated. Continue to incubate for 24-48 hours. When more than 90% of the cells have cytopathic changes, remove the culture medium and add 10ml of DNAzol. Use a 10ml pipette to suck and blow 5 times, and transfer the cell lysate to a 50ml Falcon test tube, add 5ml of 100% ethanol, and ...
Embodiment 2-4
[0167] This example is used to illustrate the recombinant type II herpes simplex virus of the present invention.
[0168] According to the same principle as the steps of Example 1, the recombinant virus HG52d34.5GFP of Example 2 (the ICP34.5 gene was deleted and the GFP expression cassette was inserted at the position of the ICP34.5 gene), the recombinant virus HG52dICP47GFPd34 of Example 3 were prepared. .5 (deleting ICP34.5 gene and ICP47 gene, and inserting GFP expression cassette at the position of ICP47 gene) and embodiment 4HG52dICP47GFPd34.5GFP (deleting ICP34.5 gene and ICP47 gene, and inserting GFP expression box at the position of ICP34.5 gene and ICP47 gene Insert the GFP expression cassette at the position).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com