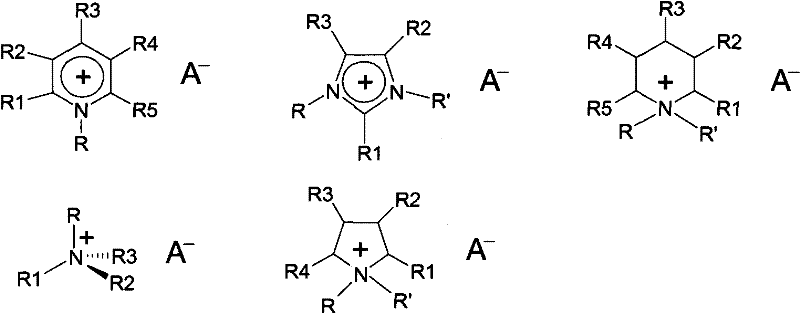
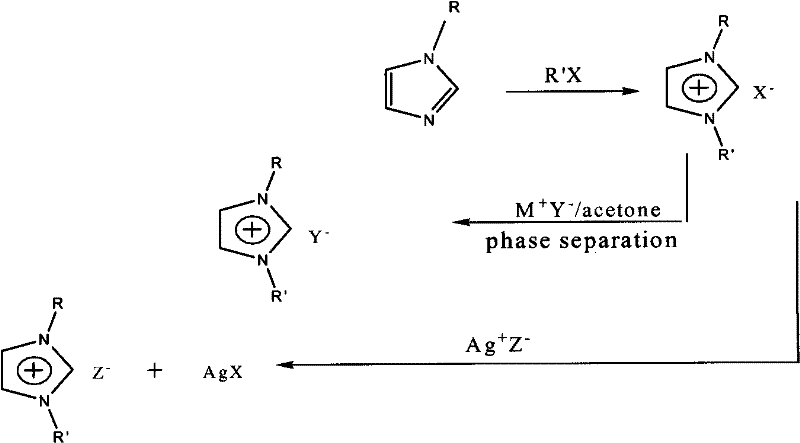
Method for synthesizing organic ionic compounds
A technology of organic ions and synthesis methods, which is applied in the fields of organic chemistry, sulfonate preparation, amino-substituted functional group preparation, etc., can solve the problems of cumbersome reaction routes, etc., and achieves improved reaction efficiency, wide melting range, improved conversion rate and selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Take by weighing a certain amount of triethylamine and tetrafluoroboric acid aqueous solution at a molar ratio of 1:1, stir at room temperature for 2 hours, then remove water by distillation under reduced pressure to obtain triethylammonium tetrafluoroborate (moisture content) with lower moisture content The content is less than 2%); Weigh triethylammonium tetrafluoroborate, dimethyl carbonate, and methanol successively in a molar ratio of 1:1.1:2, add them to the reaction kettle, heat up to 140°C, and stir at this temperature for 24 hours, and then cooled to room temperature, the yield of triethylmethyltetrafluoroborate quaternary ammonium salt was >93%.
Embodiment 2
[0053] Weigh a certain amount of N-methylimidazole and hexafluorophosphoric acid aqueous solution at a molar ratio of 1:1, stir at room temperature for 4 hours, then distill off the water under reduced pressure to obtain N-methylimidazole hexafluorophosphoric acid with a low water content Onium salt (moisture content is less than 3%); Weigh N-methylimidazolium hexafluorophosphate onium salt, dimethyl carbonate, and methanol sequentially in a molar ratio of 1:1.5:5, add it to the reaction kettle, and heat up to 140°C. Stir at this temperature for 12 hours, then cool to room temperature, the yield of 1-ethyl-3-methylimidazolium hexafluorophosphate is >80%.
Embodiment 3
[0055] Weigh a certain amount of N-methylimidazole and tetrafluoroboric acid aqueous solution at a molar ratio of 1:1, stir at room temperature for 6 hours, then distill off the water under reduced pressure to obtain N-methylimidazole tetrafluoroboric acid with a low moisture content Onium salt (moisture content is less than 3%); Weigh N-methylimidazolium tetrafluoroborate onium salt and dimethyl carbonate successively in a molar ratio of 1:3, add to the reaction kettle, heat up to 160°C, at this temperature Stir for 4 hours, then cool to room temperature, the yield of 1-ethyl-3-methylimidazolium tetrafluoroborate > 82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com