Quality control method of xiaojiean preparation
A quality control method and preparation technology, which is applied in the field of TLC identification and content determination of Smilax smilax in Xiaojie'an preparations, and can solve the problems that there are no identification methods and content determination methods for Smilax smilax
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
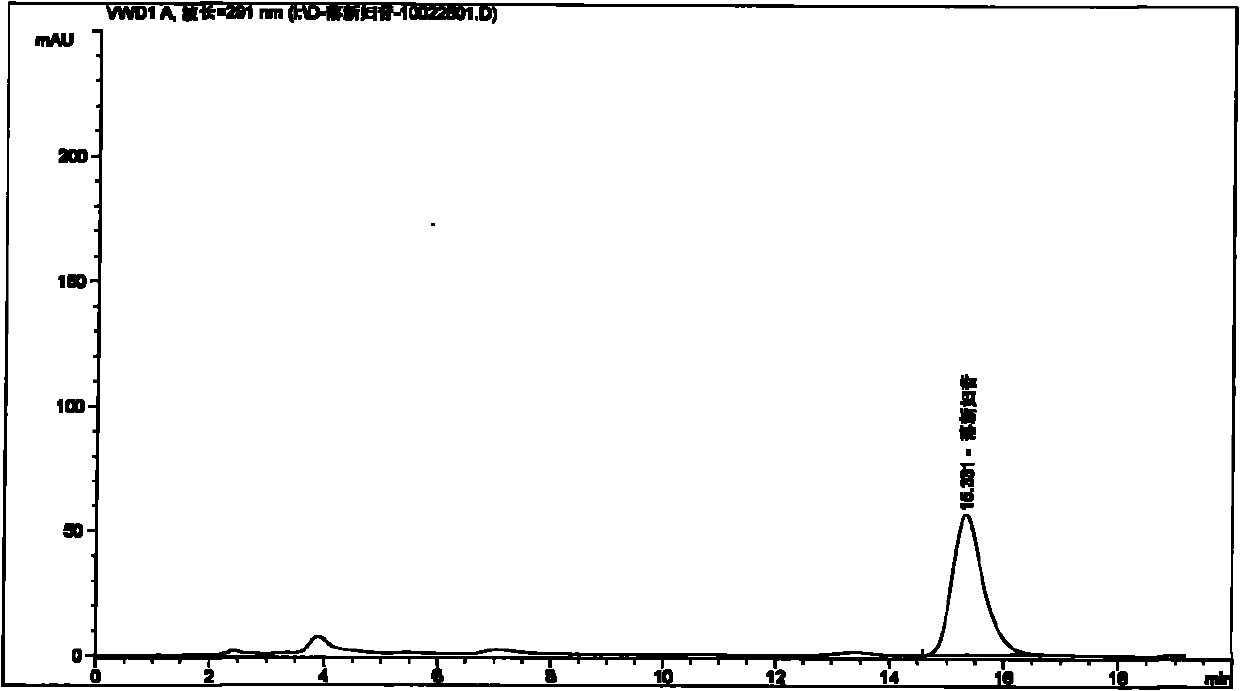
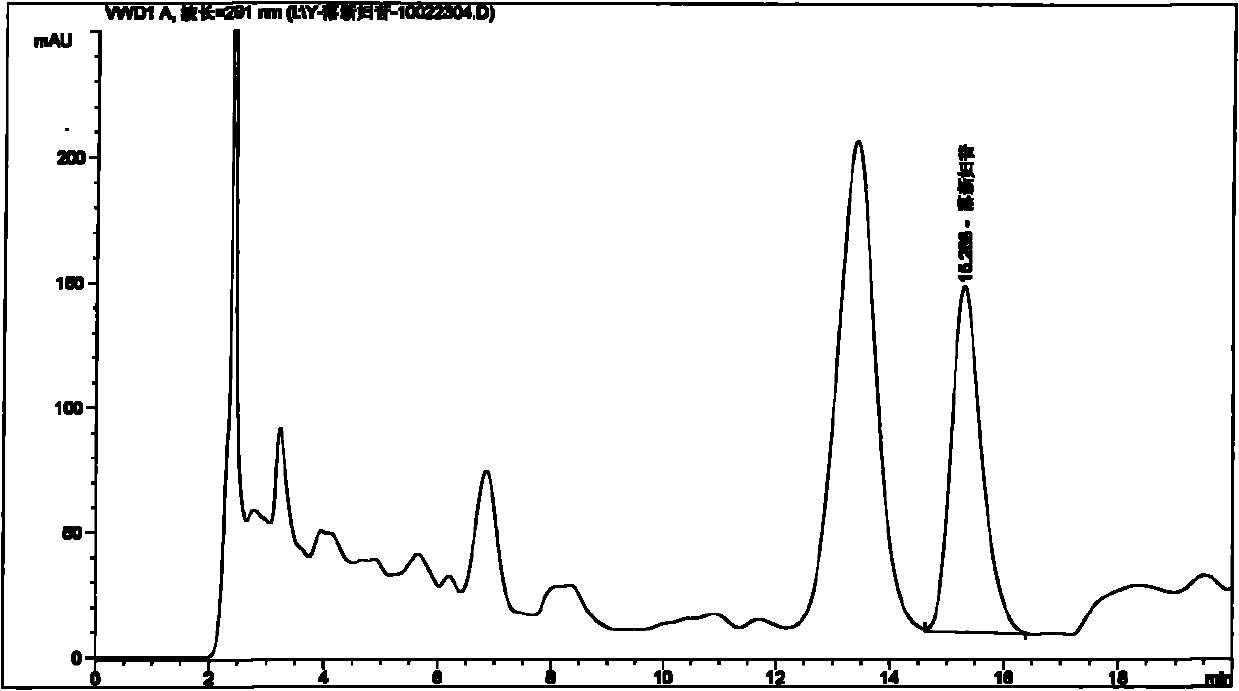
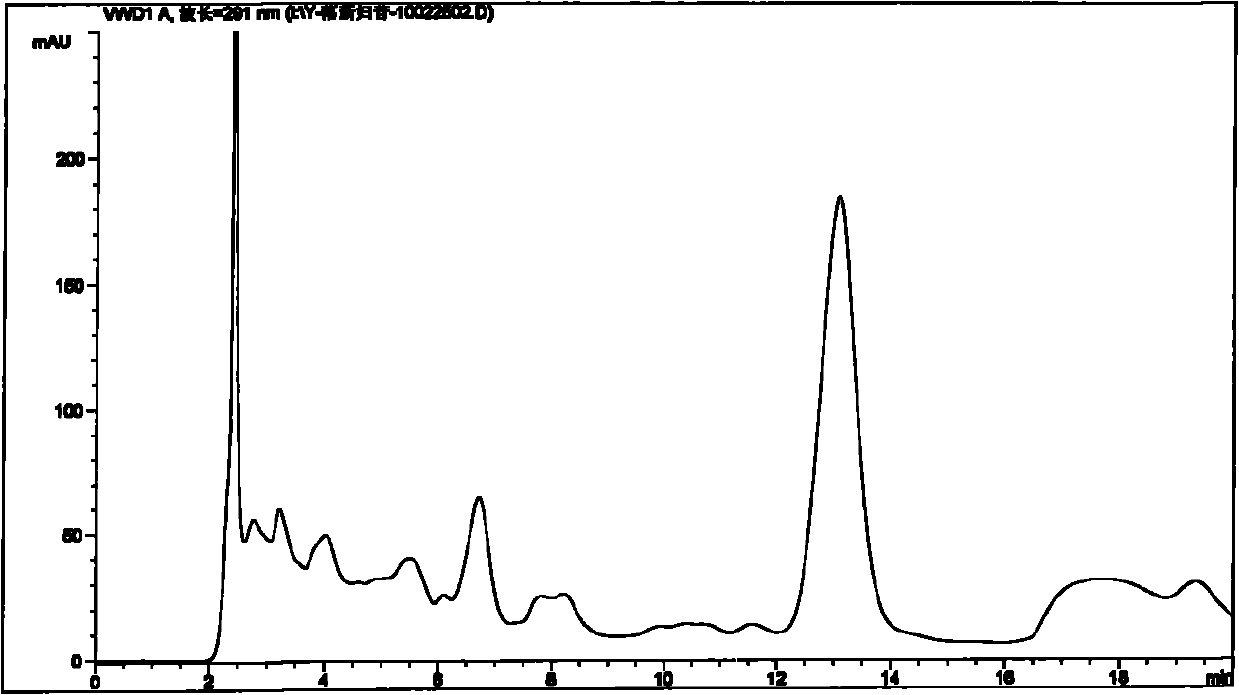
[0089] Embodiment 1: Take the test product (for example, weigh 1.5 g of the content of 09090505 Pixiaojie’an Capsule), which contains 5 mg of Astilbin; solution 10ml), the test sample contains astilbin 5mg, concentrated to dryness; add methanol 15ml to the above test sample, ultrasonically place for 15 minutes, let cool, filter, and the filtrate is concentrated to 2ml, as the test solution. Another astilbin reference substance 3mg, add methanol 1ml to dissolve, as the reference substance solution. Then take 1 g of Smilax smilax as reference medicinal material, and prepare the reference medicinal material solution in the same way. According to the thin-layer chromatography (Appendix VI B of "Chinese Pharmacopoeia") test, draw 2 μl of each of the above three solutions, respectively spot on the same silica gel G thin-layer plate, and mix with chloroform-methanol-formic acid (4:1: 0.05) as the developer, develop, take out, dry in the air, spray with 5% vanillin and 10% sulfuric a...
Embodiment 2
[0090] Embodiment 2: The color developer described in embodiment 1 is replaced with 2% ferric chloride ethanol solution, after spraying with the color developer, it is inspected under sunlight, and other is the same as embodiment 1.
Embodiment 3
[0091] Embodiment 3: The color developer described in Example 1 was replaced with 1% aluminum chloride ethanol solution, after spraying with the color developer, it was inspected under an ultraviolet lamp (365nm), and the others were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com