Aryl gpr120 receptor agonists and uses thereof
A bicyclic aryl and bicyclic heteroaryl technology, applied in the field of aryl GPR120 receptor agonists and their uses, can solve problems such as elevation and increased insulin release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0402] Preparation of compounds of the present invention
[0403] The compounds of the present invention can be prepared in a variety of ways familiar to those skilled in the art of synthetic organic chemistry. Synthetic routes to compounds of the present invention are not limited to the methods outlined herein or as provided in the Examples. Individual compounds may require manipulation of conditions to provide various functional groups and may require the appropriate use of protecting groups. Purification can be achieved, if necessary, by elution on a silica gel column with an appropriate organic solvent system. Reverse phase HPLC or recrystallization can also be used.
[0404] Therapeutic compositions and methods
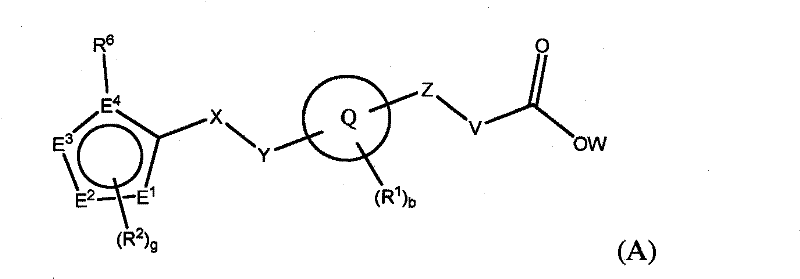
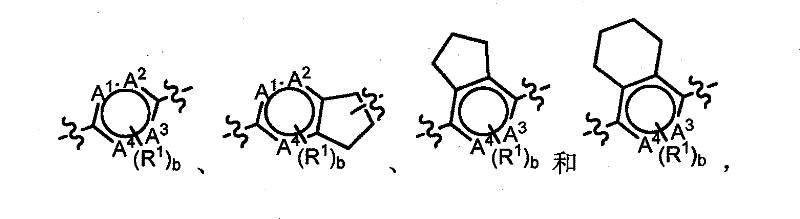
[0405] According to the present invention there is provided a method of treating a disease or condition selected from the group consisting of Type I diabetes, Type II diabetes and metabolic syndrome. The methods comprise administering to a subject in need of ...
example 1
[0648] 3-(3,5-Difluoro-4-((3-isopropyl-1-phenyl-1H-pyrazol-5-yl)methoxy)phenyl)propanoic acid (55)
[0649]
[0650] Step A: Add intermediate (53) (0.160 g, 0.693 mmol), polymer-supported triphenylphosphine (3 mmol / g, 0.347g, 1.04mmol) and diisopropyl azodicarboxylate (0.205mL, 1.04mmol). The resulting suspension was stirred for 18 hours. The reaction was diluted with ethyl acetate and filtered through a pad of celite. The filtrate was concentrated in vacuo and the residue was purified by silica gel chromatography (0-20% EtOAc in hexanes) to afford intermediate (54).
[0651] Step B: To a solution of intermediate (54) (0.270 g, 0.630 mmol) in THF (1 mL) and methanol (1 mL) was added lithium hydroxide solution (1.0 M, 1.0 mL). The reaction was stirred at room temperature for 4 hours. The mixture was acidified with 1M HCl and diluted with EtOAc (5 mL). The organic layer was washed with brine (5 mL), dried over sodium sulfate and filtered. The filtrate was concentrated i...
example 2
[0653] Ethyl 3-(3,5-difluoro-4-((4-phenyl-1,2,3-thiadiazol-5-yl)methoxy)phenyl)propanoate (57)
[0654]
[0655] Step A: To a solution of intermediate (5) (0.200 g, 0.787 mmol) in acetonitrile (3 mL) was added intermediate (53) (0.181 g, 0.787 mmol) and cesium carbonate (0.308 g, 0.945). The resulting suspension was stirred at 80°C for 4 hours. The reaction was cooled to room temperature, diluted with ethyl acetate and filtered through a pad of celite. The filtrate was concentrated in vacuo and the residue was purified by silica gel chromatography (0-20% EtOAc in hexanes) to afford intermediate (56).
[0656] Step B: To a solution of intermediate (56) (0.318 g, 0.787 mmol) in THF (1 mL) and methanol (1 mL) was added lithium hydroxide solution (1.0 M, 1.0 mL). The reaction was stirred at room temperature for 4 hours. The mixture was acidified with 1M HCl and diluted with EtOAc (5 mL). The organic layer was washed with brine (5 mL), dried over sodium sulfate and filtered....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com