Preparation method of biapenem intermediate
A technology for biapenem and intermediates, which is applied in the field of preparing compound 4-bromo-1, can solve the problems of many by-products, harsh operation requirements, poor selectivity, etc., achieve reduced reaction time, mild reaction conditions, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
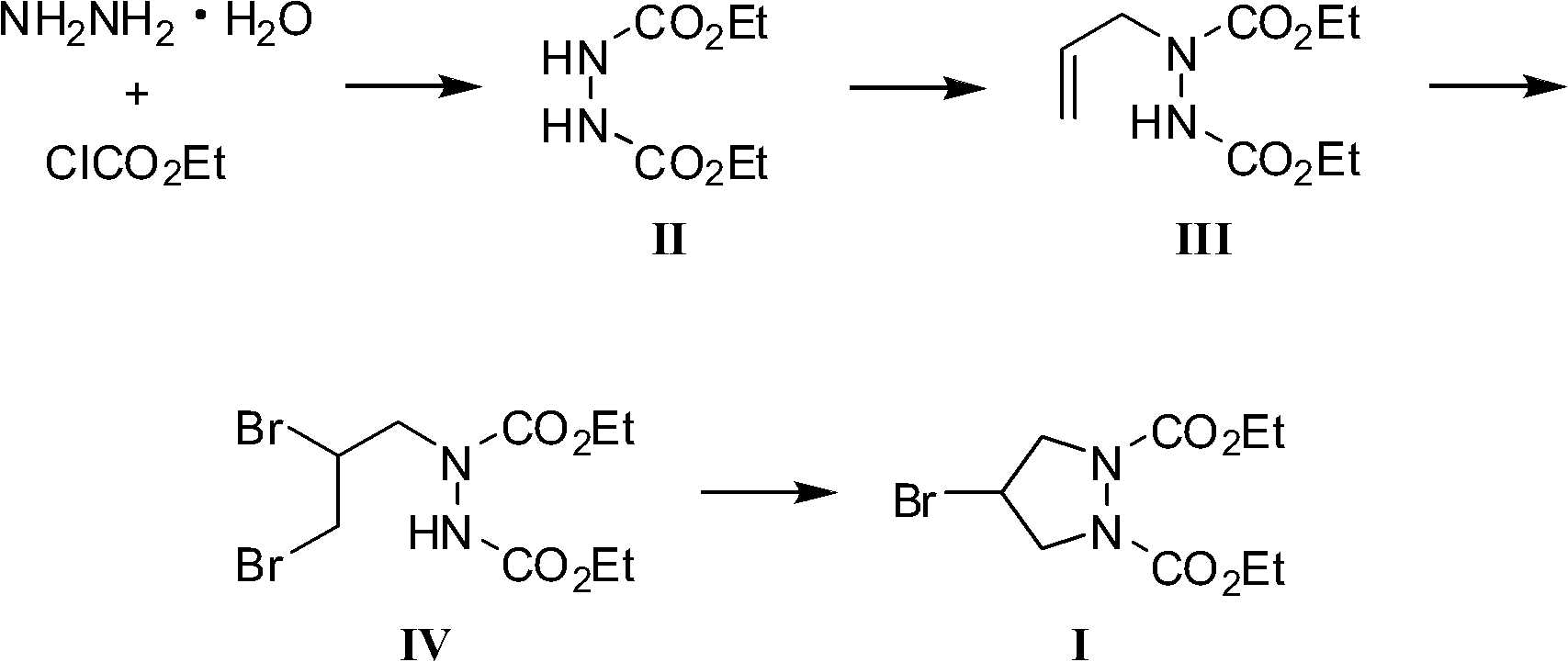
[0027] A. Preparation of 1,2-diethoxycarbonyl hydrazine hydrate (II)
[0028] In the reactor equipped with mechanical stirring, mercury thermometer, and constant pressure dropping funnel, add hydrazine hydrate 50g (1.0mol), anhydrous sodium bicarbonate 184.8g (2.2mol), water 600ml, mechanically stir to dissolve the solid, the system Mix well and cool to 0°C in an ice bath. Add 238.7 g (2.2 mol) of ethyl chloroformate into the constant pressure dropping funnel, and drop this into the reactor under stirring, and the drop is completed within 1 hour. After the dropwise addition, the ice bath was removed, and it was naturally raised to room temperature, and the stirring reaction was continued for 4 hours. After the reaction was completed, the white solid separated out was collected by filtration, washed with water and dried to obtain 170 g of white powder, which was 1,2-diethoxycarbonyl hydrazine hydrate ( II), yield 96.6%, M.P.127-128°C.
[0029] B. Preparation of 1-allyl-1,2-di...
Embodiment 2
[0036] Other steps are identical with embodiment 1, just the 1 of A step, the preparation method of 2-diethoxycarbonyl hydrazine hydrate (II) is as follows:
[0037] In a reactor equipped with mechanical stirring, a mercury thermometer, and a constant pressure dropping funnel, add 50 g (1.0 mol) of hydrazine hydrate, 168 g (2.0 mol) of anhydrous sodium bicarbonate, and 400 ml of water, stir mechanically to dissolve the solid, and mix the system homogeneous, cooled to 0°C in an ice bath. Add 217 g (2.0 mol) of ethyl chloroformate into the constant-pressure dropping funnel, and drop it into the reactor while stirring, and finish dropping within 1 hour. After the dropwise addition, the ice bath was removed, and it was naturally raised to room temperature, and the stirring reaction was continued for 4 hours. After the reaction was completed, the white solid separated out was collected by filtration, washed with water, and dried to obtain 161 g of white powder, which was 1,2-dietho...
Embodiment 3
[0039] Other steps are identical with embodiment 1, just the 1 of A step, the preparation method of 2-diethoxycarbonyl hydrazine hydrate (II) is as follows:
[0040] In the reactor equipped with mechanical stirring, mercury thermometer, and constant pressure dropping funnel, add hydrazine hydrate 50g (1.0mol), anhydrous sodium bicarbonate 176.4g (2.1mol), water 500ml, mechanically stir to dissolve the solid, the system Mix well and cool to 0°C in an ice bath. Add 227.8 g (2.1 mol) of ethyl chloroformate into the constant-pressure dropping funnel, and drop it into the reactor while stirring, and finish dropping within 1 hour. After the dropwise addition, the ice bath was removed, and it was naturally raised to room temperature, and the stirring reaction was continued for 4 hours. After the reaction was completed, the white solid separated out was collected by filtration, washed with water, and dried to obtain 167 g of white powder, which was 1,2-diethoxycarbonyl hydrazine hydra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com