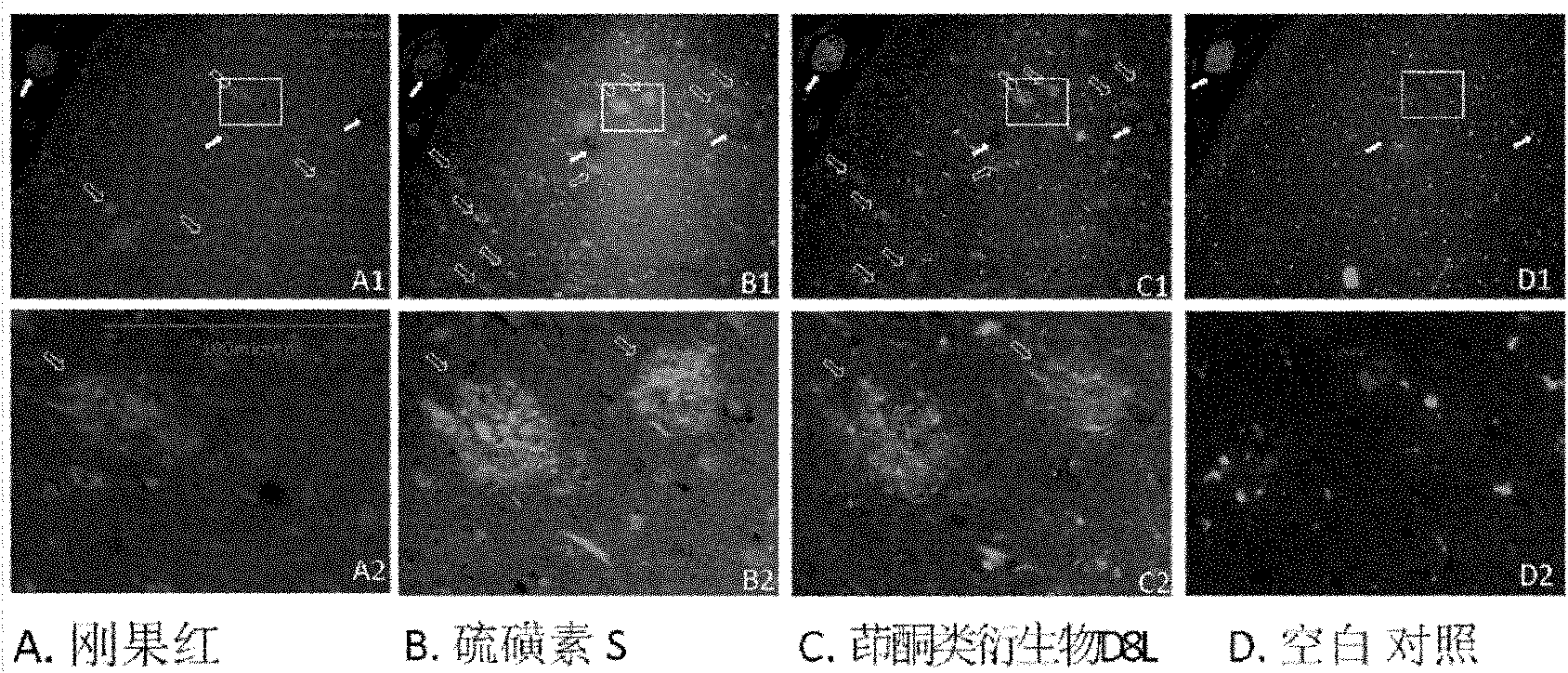
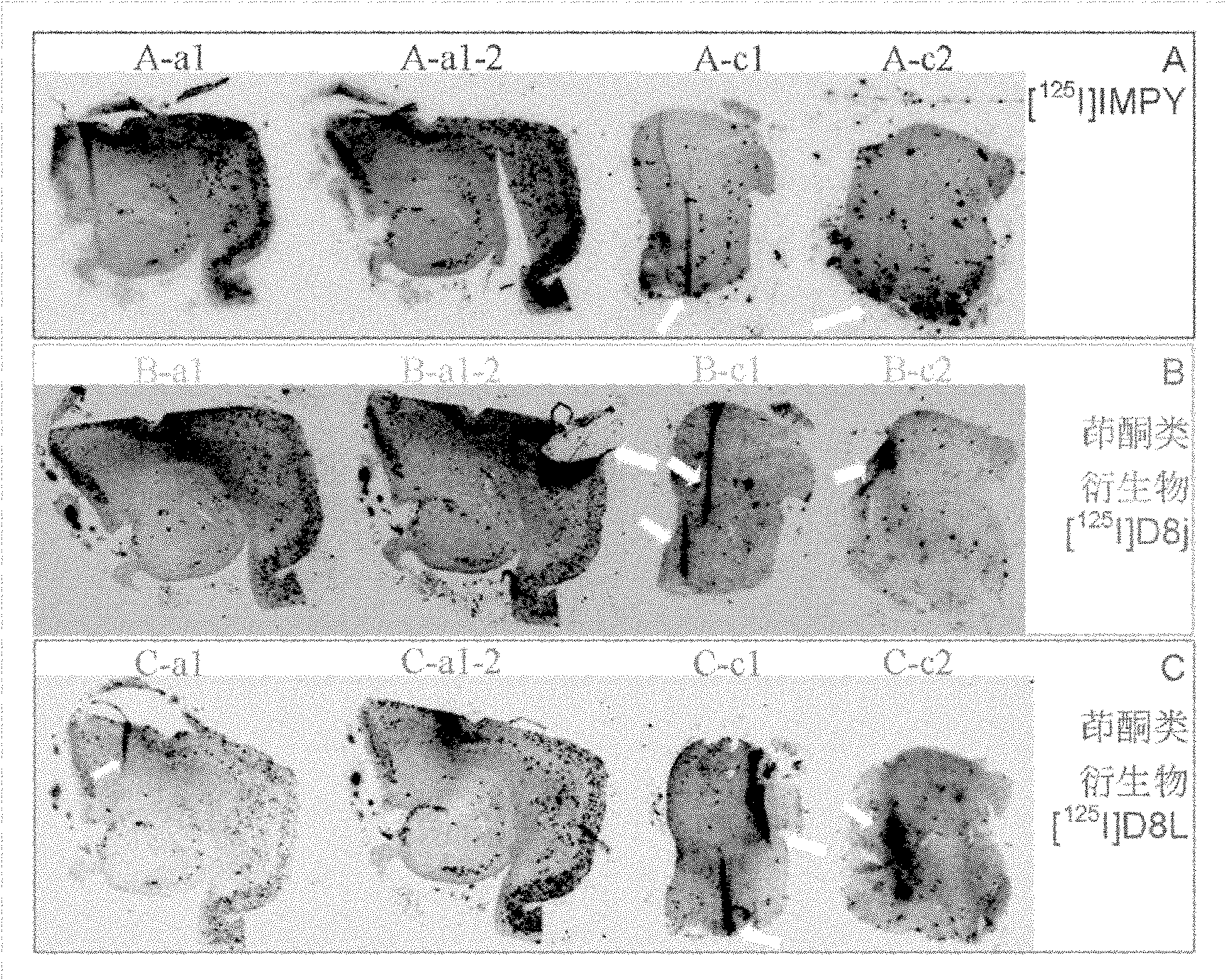
Indenone derivative and applications thereof as developing agent and aggregation inhibitor of amyloid protein deposit and neurofibrillary tangle
A compound and complex technology, applied in the field of preparation of labeled compounds, can solve the problems of slow clearance, large trauma and risk of biopsy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Example of labeling method of radioactive isotope
[0058] 1. 11 C mark (shown in the following reaction formula):
[0059]
[0060] In a 3 mL V-shaped flask, 1 mg (0.0036 mmol) of compound 2-(4-dimethylamino-benzylidene)-5-hydroxy-1-indanone was dissolved in 400 μL DMSO, 10 mg dry KOH was added, and vortexed for 5 min. [ 11 C]CH 3 I was bubbled through the solution at 30 mL / min, and the reaction was heated at 95 °C for 5 min. Then, the reaction product was purified by semi-preparative HPLC, Prodigy ODS column, eluent was 70% acetonitrile / 30% triethylamine phosphate buffer (pH7.2), collected containing 11 C labeled radioactive compound.
[0061] 2. 125 I mark (shown in the following reaction formula):
[0062]
[0063] Sequentially weigh the compound 2-(4-dimethylamino-benzylidene)-5-bromo-1-indanone 400mg (1.17mmol), bistributyltin 4.0g (7.02mmol) and tetrakistriphenylphosphine palladium Pd(PPh 3 ) 4 135mg (0.12mmol), add 8mL dioxane and 2mLe...
Embodiment 2
[0070] Embodiment 2: Design example of complexes
[0071] 1. Ligand example:
[0072]
[0073] In the ligand structure shown in the above structural formula (1-4), a multi-dentate coordination site is constructed on the benzene ring. This coordination site can be located at other positions of the benzene ring, and can also be moved to the benzene ring connected to indanone superior. In this multidentate coordination region, single bonds can be replaced by carbon-carbon double bonds or triple bonds, and double bonds can also be reduced to single bonds. The heteroatoms therein can be O, N, S, P.
[0074] Design example of ferromagnetic complex (shown in the following reaction formula)
[0075]
[0076] Taking the above ligand 1 as an example, prepare the Mn complex (as shown in the following reaction formula)
[0077]
[0078] In 20mL of DMF, add bromoethanol 375mg (3mmol), (E)-2-(4-dimethylamino-benzylidene)-5,6-diamino-1-indanone 293mg (1mmol) and carbonic acid P...
Embodiment 3
[0081] A method for synthesizing a series of derivatives using indanone as a raw material, wherein D8a-c, D8e-h, D8l and D8m can be easily obtained by base catalysis, and the operation is simple and the yield is high. D8d can be obtained by reducing D8c under the catalysis of SnCl2 2H2O, and D8h and D8j can be refluxed with (Bu3Sn)2 under the catalysis of Pd(PPh3)4 in triethylamine to obtain tributyltin-substituted products D8i and D8k. D8i reacts with iodine in THF to obtain iodo product D8j. The reaction process is shown in the following reaction formula:
[0082]
[0083] (E)-2-Benzylidene-5,6-dimethoxy-1-indanone (D8a). Weigh 192 mg (1 mmol) of 5,6-dimethoxy-1-indanone and 106 mg (1 mmol) of benzaldehyde in 10 mL of ethanol, add 2.2 mL of 10% NaOH aqueous solution dropwise under stirring, stir overnight at room temperature, and precipitate a large amount of yellow precipitation. After suction filtration, the filter cake was recrystallized from ethanol (yield 86%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com