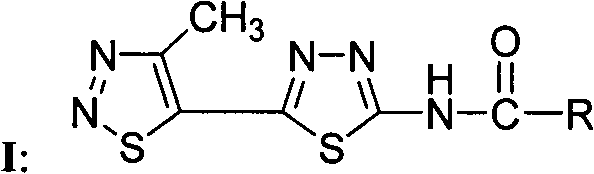
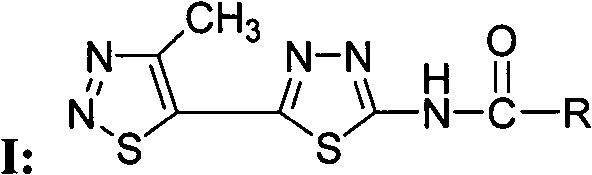
1,2,3-thiadiazole-1,3,4-thiadiazole compounds and preparation method and application thereof
A technology of thiadiazoles and thiadiazoles, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
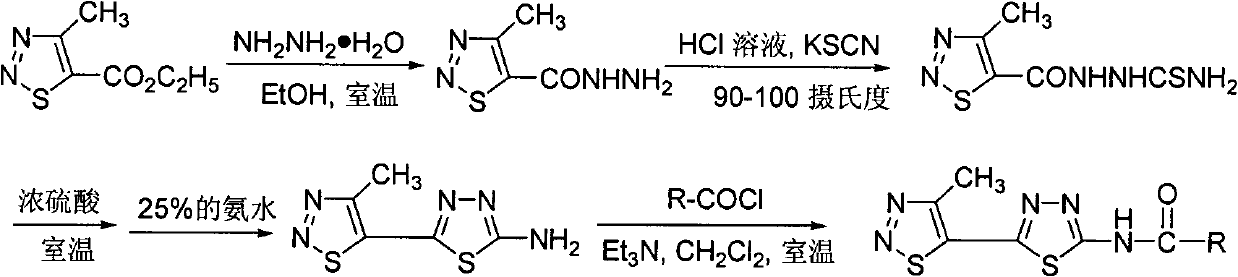
[0036] Compound WSX-9: Preparation and structure identification of 1-(4-methyl-1,2,3-thiadiazole-5-carbonyl)thiosemicarbazide
[0037] In a 100 mL round bottom flask, add 4-methyl-1,2,3-thiadiazole-5-carboxhydrazide (5 g, 31.6 mmol), concentrated hydrochloric acid (6.8 g), water (30 mL) and dry potassium thiocyanate (4.6 grams, 47.5 mmoles), heated in an oil bath at 90 to 100 degrees Celsius under electromagnetic stirring, and after about 1 hour, a large amount of white solids were precipitated, continued heating for 3 hours, stopped the reaction, and pumped out after cooling filtered, and washed with dilute sodium bicarbonate solution and water respectively, and dried to obtain a pure white solid product with a yield of 85%; melting point: 183-185 degrees Celsius; 1 Determination of HNMR, 1 HNMR (solvent: DMSO-dd 6 ), chemical shifts: 12.45(s, 1H, CONH), 9.03(s, 1H, CSNH), 7.68(s, 2H, NH 2 ), 2.93 (s, 3H, thiadiazolyl-CH 3 ).
Embodiment 2
[0039] Compound WSX-10: Preparation and structure identification of 2-amino-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-thiadiazole
[0040] Add 1-(4-methyl-1,2,3-thiadiazole-5-carbonyl)thiosemicarbazide (3.6 g, 16.6 mmol) and concentrated sulfuric acid (10 ml) into a 100 ml round bottom flask, room temperature Stir under low pressure for 6 hours, then pour the reaction solution into 80 ml of ice water, adjust the pH value to 9-10 with 25% ammonia water, a large amount of yellow solids are precipitated, after standing, they are suction filtered, washed with water and ethanol respectively, and dried Obtain pure product, yield 90%; Melting point: 246-248 degree centigrade; Carry out 1 Determination of HNMR, 1 HNMR (solvent: DMSO-d 6 ), chemical shift: 7.87(s, 2H, NH2 ), 2.85(s, 3H, thiadiazolyl-CH 3 ).
Embodiment 3
[0042] Compound WSX-59: N-[5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-thiadiazol-2-yl]-4methyl- Preparation and Structure Identification of 1,2,3-Thiadiazole Carboxamide
[0043] In a 100 ml round bottom flask was added 2-amino-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-thiadiazole (0.21 g, 1.1 mmol ), dichloromethane (15 ml) and triethylamine (0.22 g, 2.2 mmol), 4-methyl-1,2,3-thiadiazole-5-carbonyl chloride (0.27 g, 1.5 mmol) Dissolve in dichloromethane (5 ml), slowly drop into the reaction solution under stirring at room temperature, after dropping, continue to react at room temperature for 5 hours, TLC board monitors that the raw materials have been reacted, and obtain a yellow solid by suction filtration, the mother liquor is concentrated, suction filtration, and combined The solid was washed with dilute hydrochloric acid, water and methanol respectively, and the pure product was obtained after drying, with a yield of 60%; melting point: 240 degrees Celsius (decomposition); ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com