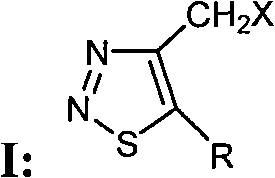
4-halogenated methyl-1,2,3-thiadiazole compounds and preparation method and application thereof
A technology of thiadiazoles and halomethyl, which is applied in the field of 4-halomethyl-1, can solve the problems of rare pesticide varieties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
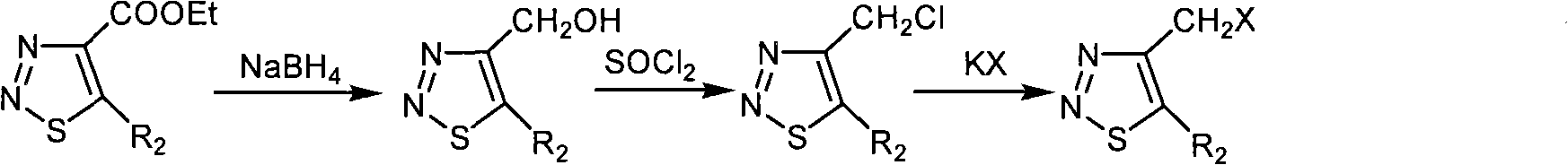
[0045] Preparation and structure identification of compound 4-chloromethyl-1,2,3-thiadiazole-5-carboxylic acid ethyl ester (ZHH-15)
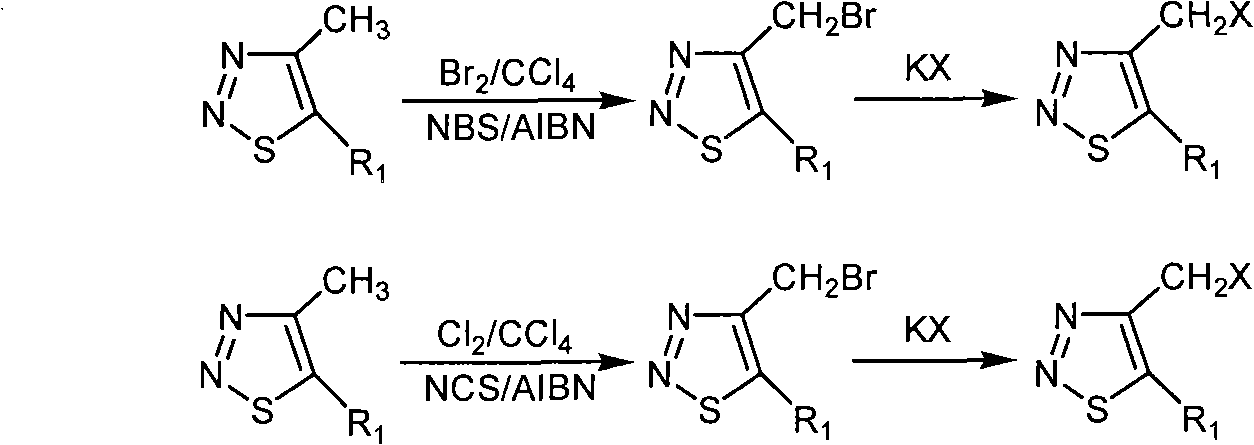
[0046] Add 10 ml of carbon tetrachloride to a 50 ml round bottom flask, then add ethyl 4-methyl-1,2,3-thiadiazole-5-carboxylate (0.67 g, 3.90 mmol), N-chloro Succinimide (NCS) (0.80 g, 5.99 mmol), azobisisobutyronitrile (AIBN) (0.04 g, 0.24 mmol), the reaction system was refluxed for 48 hours under stirring, and after the reaction was finished, filter Product, the solid is washed with 10 ml of carbon tetrachloride × 3, the organic phases are combined, and the solvent is removed under reduced pressure with 200-300 mesh silica gel column chromatography, the eluent is petroleum ether at 60-90 degrees Celsius: ethyl acetate, volume Ratio is 40: 1, calculates yield with gained pure product, yield 26%, carry out 1 Determination of HNMR, 1 HNMR (solvent: CDCl 3 ), chemical shift: 5.282 (s, 2H, CH 2 ), 4.493-4.439 (q, 2H, J=7.2Hz, J=14.4Hz, CH 2 ),...
Embodiment 2
[0049] Preparation and structure identification of the compound 4-bromomethyl-1,2,3-thiadiazole-5-carboxylic acid ethyl ester (ZHH-1)
[0050] Add 10 ml of carbon tetrachloride to a 50 ml round bottom flask, then add 4-methyl-1,2,3-thiadiazole-5-carboxylic acid ethyl ester (0.61 g, 3.55 mmol), N-bromo Succinimide (NBS) (0.72 grams, 4.04 millimoles), azobisisobutyronitrile (AIBN) (0.05 grams, 0.30 millimoles), the reaction system was refluxed for 8 hours under stirring, and after the reaction was finished, filter Product, the solid is washed with 10 milliliters of carbon tetrachloride × 3, the organic phases are combined, the organic phase is washed 3 times with sodium thiosulfate solution, dried with anhydrous sodium sulfate after washed 3 times with distilled water, and then evaporated under reduced pressure to remove the solvent with 200 -300 mesh silica gel column chromatography, the eluent is sherwood oil of 60-90 degrees centigrade: ethyl acetate, the volume ratio is 40: ...
Embodiment 3
[0053] Preparation and structure identification of compound 4-iodomethyl-1,2,3-thiadiazole-5-carboxylic acid ethyl ester (ZHH-13)
[0054] Add 4-bromomethyl-1,2,3-thiadiazole-5-ethyl carboxylate (0.30 g, 1.2 mmol) in a 50 ml round bottom flask, add 15 ml of solvent acetone, then add potassium iodide (0.30 g, 1.8 mmol), stirred at room temperature for 22 hours, filtered out the solid in the solution, and the filtrate decompressed and evaporated the solvent to obtain 4-iodomethyl-1,2,3-thiadiazole- 5-Ethyl formate, the eluent is petroleum ether at 60-90 degrees centigrade: ethyl acetate, the volume ratio is 10: 1, and the yield is calculated with the pure product obtained; the yield is 64%, carried out 1 Determination of HNMR, 1 HNMR (solvent: CDCl 3 ), chemical shift: 5.119 (s, 2H, CH 2 ), 4.488-4.435 (q, 2H, J=7.2Hz, J=14.4Hz, CH 2 ), 1.454-1.419 (t, 3H, J=7.2Hz, CH 3 ), the compound's 1 H NMR data show that it is consistent with its chemical structure; its physical and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com