Hdrogenation synthesis method for preparing 2-methyl allyl alcohol by using recyclable catalyst
A technology of methacryl alcohol and methacrolein, which is applied in the field of organic intermediate synthesis, can solve the problems of large amount of by-product ether generation, difficult to control, low yield, etc., achieve simple recycling method and reduce separation cost , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
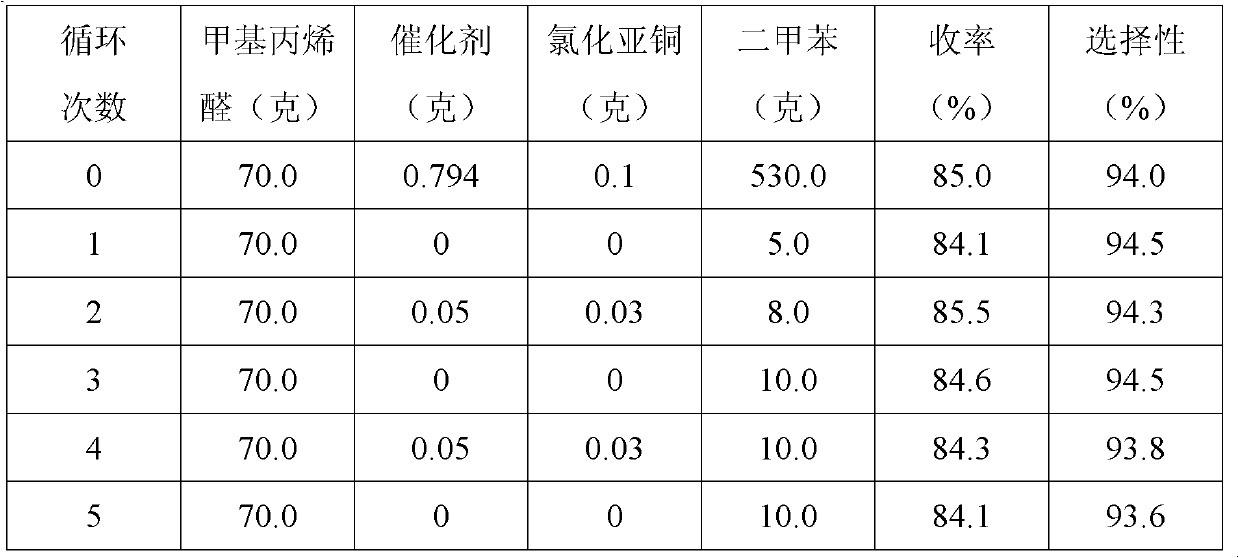
[0031] 1. Add 70 grams of 2-methacrolein and 0.794 grams of Ru[(±)-BINAP]Cl to a 1-liter autoclave 2 , 0.1 gram cuprous chloride and 530 gram xylenes, airtight autoclave, pass into hydrogen twice in the autoclave to replace the original air in the autoclave; After replacement, lead the hydrogen of 3.5MPa in the autoclave, and 30 Reaction at ℃ for 6 hours;
[0032] 2. After the reaction is over, cool the reaction system to room temperature, discharge the remaining hydrogen, open the autoclave, and transfer the reaction solution to a 1-liter three-port still, and install a 15-plate fine distillation still on the still. Distillation column, heating the still, so that the temperature of the still rises gently from room temperature to 150 ° C, when there is reflux at the top of the tower, control the reflux ratio to 1.5, collect and obtain 5 grams of fractions at 30 to 80 ° C, 2-methacrolein The content is 85%, the fraction of 80-100°C is 3 grams, the content of 2-methacrolein is ...
Embodiment 2
[0037] 1. Add 70 grams of 2-methacrolein and 0.794 grams of Ru[(±)-BINAP]Cl to a 1-liter autoclave 2 , 0.05 grams of cuprous chloride and 600 grams of N, N-dimethylacetamide, sealed autoclave, into the autoclave twice hydrogen to replace the original air in the autoclave; 2.5MPa hydrogen, and react at 20°C for 8 hours;
[0038] 2. After the reaction is over, cool the reaction system to room temperature, discharge the remaining hydrogen, open the autoclave, and transfer the reaction solution to a 1-liter three-port distillation kettle, and install a 18-plate fine distillation kettle on the distillation kettle. Distillation column, heating the still, so that the temperature of the still rises gently from room temperature to 150 ° C, when there is reflux at the top of the tower, control the reflux ratio to 2.0, collect and obtain 4 grams of fractions at 30 to 80 ° C, 2-methacrolein The content is 88%, the fraction of 80-100°C is 4 grams, the content of 2-methacrolein is 90%, the...
Embodiment 3
[0041] 1. Add 140 grams of 2-methacrolein and 0.16 grams of Ru[(±)-BINAP]Cl to a 2-liter autoclave 2 , 0.1 gram of cuprous chloride and 1200 gram of dimethylbenzenes, airtight autoclave, pass into hydrogen twice in the autoclave to replace the original air in the autoclave; After replacement, lead the hydrogen of 4.5MPa in the autoclave, and 30 Reaction at ℃ for 10 hours;
[0042] 2. After the reaction is over, cool the reaction system to room temperature, discharge the remaining hydrogen, open the autoclave, and transfer the reaction solution to a 2-liter three-port distillation kettle, and install a 20-plate fine distillation kettle on the distillation kettle. Distillation column, heating the still, so that the temperature of the still rises gently from room temperature to 150 ° C, when there is reflux at the top of the tower, control the reflux ratio to 1.0, collect 12 grams of 30-80 ° C fractions, 2-methacrolein The content is 84%, the 80-100°C fraction is 5 grams, the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com