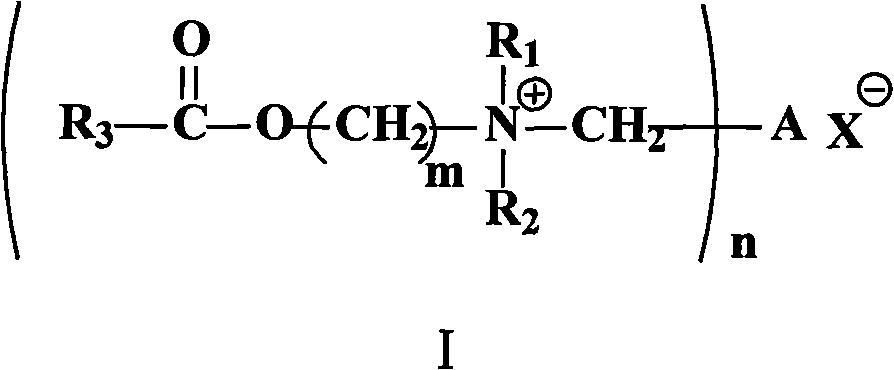
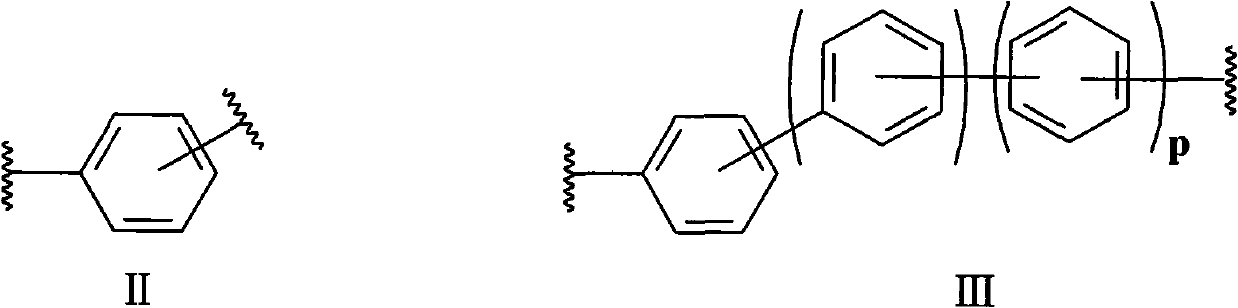
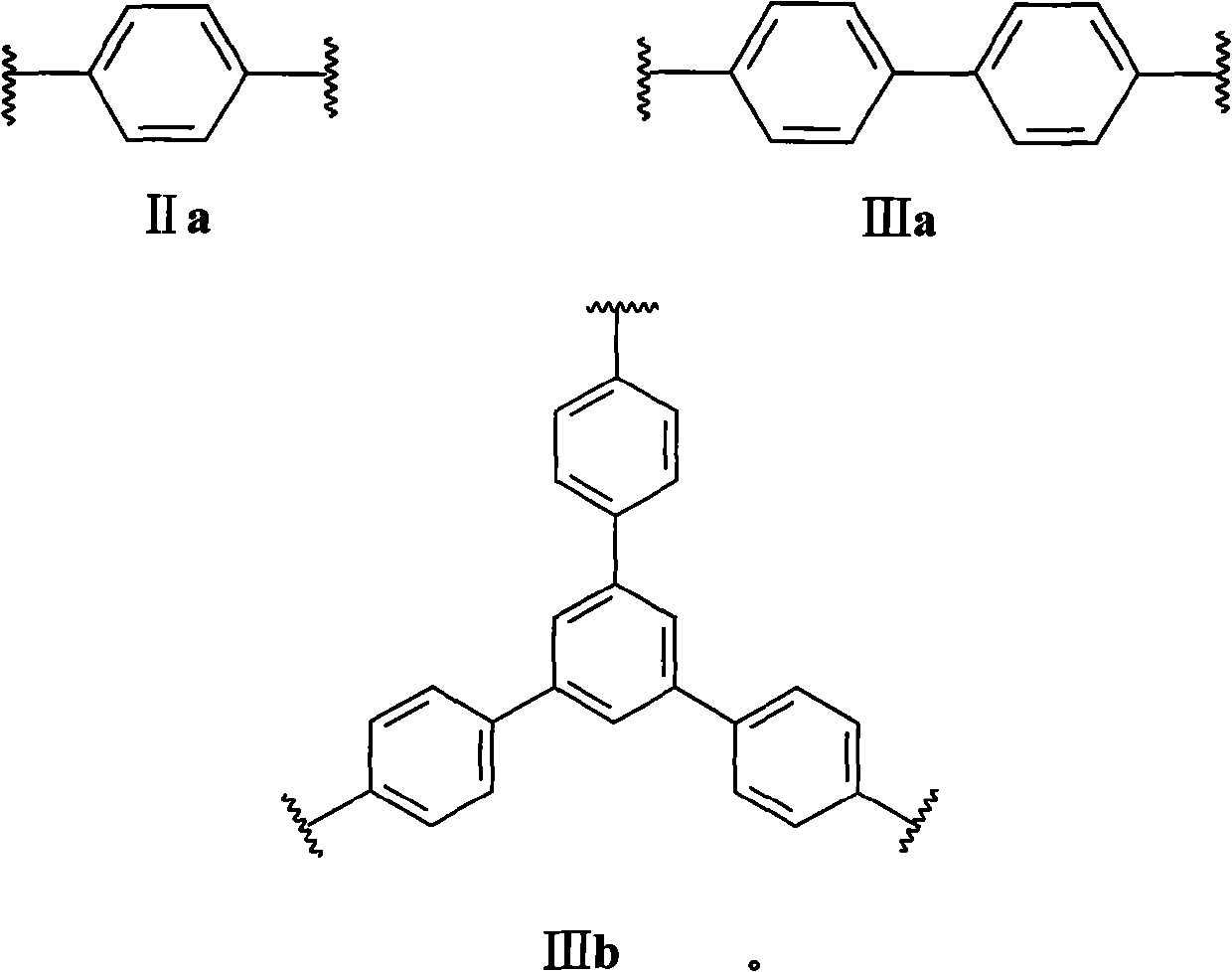
Degradable cationic Gemini surface active agent
A surfactant, cationic technology, applied in the directions of dissolution, biocide, bactericide, etc., can solve the problems of unfriendly environment, drug resistance, refractory degradation, etc., and achieve the effect of environmental friendliness, easy biodegradation, and good sterilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The method for the compound shown in the preparation formula I provided by the invention, its synthetic route is as follows:
[0024]
[0025] Specifically include the following steps:
[0026] (1) Put the aliphatic carboxylic acid (compound shown in formula IV) and hydroxyl-containing tertiary amine (compound shown in formula V) in a molar ratio of 1: (1.5~2.0) in the reactor, add catalyst, in reflux state Keep for 8 hours to 15 hours to obtain an intermediate (compound shown in formula VI); and
[0027] (2) The compound shown in formula VI and the corresponding halogenated hydrocarbon are placed in another reactor in a molar ratio of (2 to 3): 1, and ethyl acetate is used as the reaction solvent, and kept in a reflux state for at least 48 hours, The target compound (compound represented by formula I) was obtained.
[0028] The cationic surfactant designed and prepared by the invention has good bactericidal and bacteriostatic properties to Staphylococcus aureus, E...
Embodiment 1
[0032] The synthesis of cationic surfactant shown in formula I-1
[0033]
[0034] (1) Synthesis of long-chain ester group tertiary amine:
[0035] Add 0.01mol dodecanoic acid and 0.02mol hydroxyl-containing tertiary amine (compound shown in formula V, R 1 and R 2 Both CH 3 , m=2), add 1-3‰ p-toluenesulfonic acid and 50ml xylene, reflux reaction for 10h, judge the reaction process with a water trap, stop the reaction when anhydrous is generated, and fractionate to obtain a long-chain ester group tertiary amine;
[0036] (2) Synthesis of target object (compound shown in formula I-1):
[0037] Add 0.02mol and 0.01mol of the long-chain ester-based tertiary amine prepared in the step (1) into the three-necked flask, add 50ml of ethyl acetate as a solvent, and react at room temperature for 48h to obtain the crude product Recrystallized from dichloromethane and ethyl acetate to obtain the target compound (compound represented by formula I-1).
[0038] 1 H NMR (CDCl 3 , 400...
Embodiment 2
[0040] The synthesis of cationic surfactant shown in formula I-2
[0041]
[0042] Add 0.02mol of long-chain ester group tertiary amine and 0.01mol of 1,4-dibromobutene-2 in the three-necked flask, and add 50ml of ethyl acetate as a solvent, react at room temperature for 48h, and recrystallize the crude product. The obtained crude product was recrystallized from dichloromethane and ethyl acetate to obtain the target compound (compound represented by formula I-2).
[0043] 1 H NMR (CDCl 3 , 400MHz): δ6.64(s, J=8.0Hz, 2H), 5.08(d, J=4.8Hz, 4H), 4.58(d, J=2.0Hz, 4H), 4.13(s, 4H), 3.48 (s, 16H), 2.37 (t, J = 7.6Hz, 4H), 1.23 (s, 32H), 0.88 (t, J = 6.8Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com