Nasal administration preparation and application thereof
A nasal administration preparation and preparation technology, which is applied in the field of chemical pharmacy, can solve the problems of low oral effect, achieve the effects of accurate dosage, easy use, and increased transport speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1 (nasal drops / nasal spray)
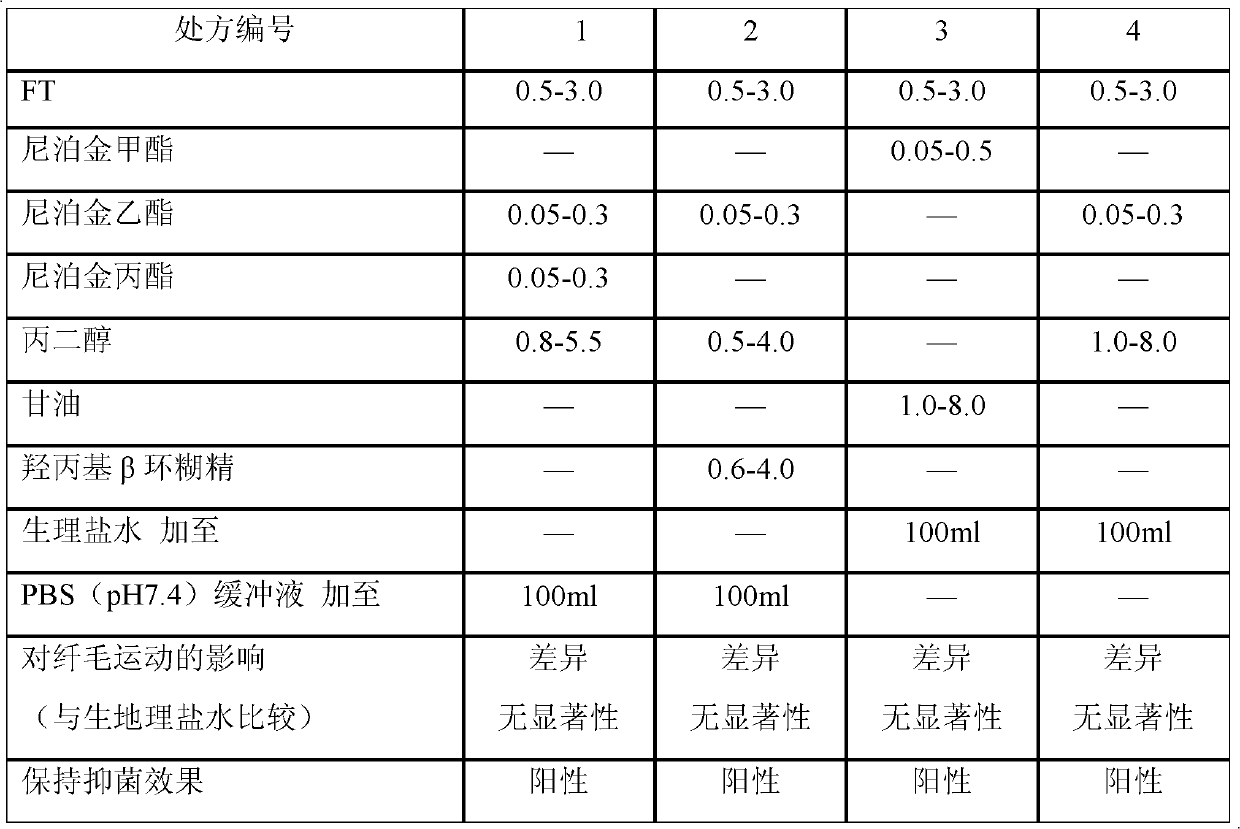
[0070] FT 2.0g, paraben complex ester (respectively weigh an appropriate amount of paraben ethyl and propyl paraben, and mix the two at a ratio of 1:1 to obtain a paraben complex ester, the same below) 0.15g, Propylene glycol 3.5g, PBS (pH7.4) buffer (that is, phosphate buffer (pH7.4): take 1.36g of potassium dihydrogen phosphate, add 0.1mol / L sodium hydroxide solution 79ml, dilute to 200ml with water, that is obtained. The same below) was added to 100ml.
[0071] Preparation method: Weigh 2.0g FT and dissolve it with 1.7ml PBS (pH7.4) buffer solution, pour it into the preparation tank, add 3.5g propylene glycol, stir, let it stand, filter, and then use buffer solution PBS (pH7.4) in the filtrate ) buffer solution to a final volume of 100ml, and finally add paraben complex ester, stir evenly, let stand, filter, potting, and sterilize to obtain.
Embodiment 2
[0072] Embodiment 2 (nasal drops / nasal spray)
[0073] Add 2.0 g of FT, 0.2 g of ethylparaben, 2.0 g of propylene glycol, 2.50 g of hydroxypropyl β-cyclodextrin, and add PBS (pH7.4) buffer solution to 100 ml.
[0074] Weigh 2.0g of FT and 2.5g of hydroxypropyl β-cyclodextrin, dissolve them with 1.7ml and 4.17ml of PBS (pH7.4) buffer solution respectively, inject them into the preparation tank, add 2.0g of propylene glycol, stir, stand still, and filter. Then add PBS (pH7.4) buffer solution to the filtrate to a final volume of 100ml, and finally add ethylparaben, stir well, let stand, filter, pot, and sterilize to obtain.
Embodiment 3
[0075] Embodiment 3 (nasal drops / nasal spray)
[0076] Add FT 2.0g, methyl paraben 0.25g, glycerin 4.2g, and normal saline to 100ml.
[0077]Weigh 2.0g of FT and dissolve it with 1.85ml of normal saline, inject it into the preparation tank, then add 4.2g of glycerin, stir, let stand, filter, add the filtrate to the final volume of 100ml with normal saline, finally add methylparaben, stir Mix evenly, let stand, filter, pot, sterilize, and you get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com