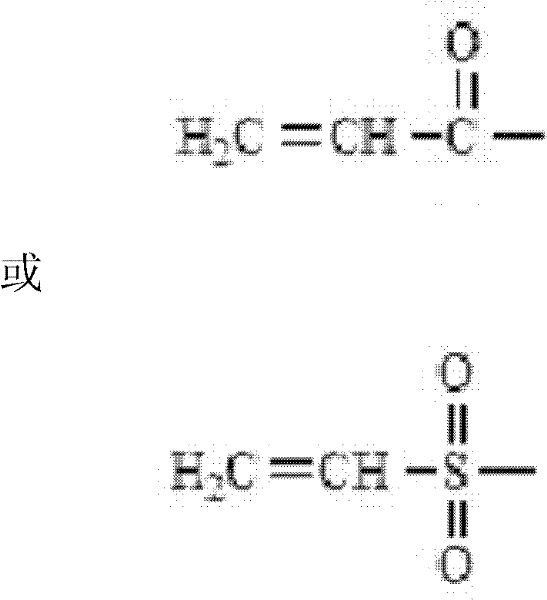
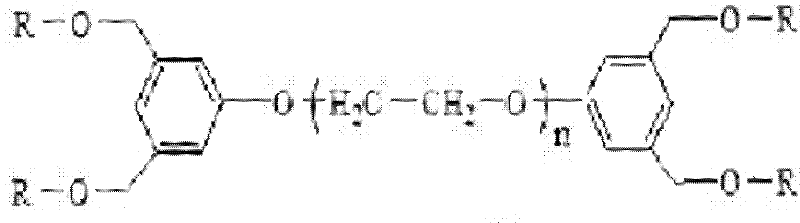Functional modification method for branched polyethylene glycol (PEG) derivative
A polyethylene glycol, branching technology, applied in the fields of biomedicine and protein chemistry, to achieve the effect of improving biological properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 5g dry PEG 3400 Dissolve in 50 mL of anhydrous dichloromethane, add 3.34 mL of triethylamine and 0.45 mL of methanesulfonyl chloride under the protection of argon, and react at room temperature for 12 hours (the reaction process is shown in the figure below). The reaction solution was washed three times with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, recrystallized with glacial ether, and filtered. The filtered solid was washed three times with glacial ether and dried in vacuo to obtain 5.17 g of a white powdery substance. The substance was tested by infrared spectroscopy and 1 H nuclear magnetic resonance detection and analysis, the results are as follows: IR (NaCl): 2883, 1467, 1280, 1114, 1061, 946, 842cm -1 ; 1 H NMR (CDCl 3 ): 2.84 (6H, CH 3 ), 3.63-3.75 ((OCH 2 CH 2 )n), 3.85-3.90 (4H, CH 2 OSO 2 ). Analysis of the results confirmed that the white powder was polyethylene g...
Embodiment 2
[0026] Dissolve 5.0 g of dry polyethylene glycol methanesulfonate in 50 mL of dimethylformamide, and add 2.74 g of Cs respectively under the protection of argon 2 CO 3 With 1.76g methyl 5-hydroxyisophthalate, react at room temperature for 24h, the reaction process is as follows:
[0027]
[0028] Dimethylformamide was distilled off under reduced pressure, the residue was dissolved in 50 mL of dichloromethane, and insoluble matter was removed by filtration. The filtered solid was washed three times with glacial ether and dried in vacuo to obtain 5.31 g of white powder. The substance was tested by infrared spectroscopy and 1 H nuclear magnetic resonance detection and analysis, the results are as follows: IR (NaCl): 2885, 1725, 1466, 1280, 1111, 963, 842, 760cm -1 ; 1 H NMR: 3.60-3.96 (CH 3 , (OCH 2 CH 2 )n), 4.22 (4H, CH 2 OAr), 7.77 (4H, Ar), 8.28 (2H, Ar). Analysis of the results confirmed that the white powder substance was bis(3,5-dimethoxycarbonyl)phenyl polyeth...
Embodiment 3
[0030] Take 4.85g of dry bis(3,5-dimethoxycarbonyl)phenyl polyethylene glycol and dissolve in 50mL of anhydrous tetrahydrofuran, slowly add 0.29g of lithium aluminum hydride (LiAlH 4 ), stirred overnight, the reaction equation is as follows:
[0031]
[0032] Slowly add 2.5 mL of 10% HCl dropwise under ice-bath stirring, filter, and wash with dichloromethane three times. The washed filtrate was concentrated under reduced pressure to obtain a white solid, which was then dissolved in dichloromethane, washed three times with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, recrystallized with glacial ether, and filtered. The filtered solid was washed three times with glacial ether and dried in vacuo to obtain 4.36 g of white powder. The substance was tested by infrared spectroscopy and 1 H nuclear magnetic resonance detection and analysis, the results are as follows: IR (NaCl): 3459, 2882, 1467, 1280, 1115, 946, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com