Anti-infective formulation and methods of use
A preparation, mammary gland technology, applied in the direction of anti-infective drugs, topical antibacterial agents, pharmaceutical formulations, etc., can solve problems such as impact, animal injury, infection of mammary glands, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0132] Example 1: Formulation and production - no thickener
[0133] The following table is an overview of the embodiments of the formulation
[0134] Table 1 - Formulation Overview
[0135]
[0136] As described in Table 1, the key difference between Formulation 1 and Formulation 2 is the amount of chlorhexidine (CHX) added.
[0137] Below is the manufacturing process of producing above-mentioned preparation:
[0138] 1. Take heavy liquid paraffin and aluminum stearate (aluminum stearate);
[0139] 2. Add aluminum stearate to the liquid paraffin, then stir until a smooth suspension without lumps;
[0140] 3. Heat the mixture obtained in step 2 until the temperature rises to 150°C;
[0141] 4. Keep at 150°C for 1 hour (sterilization);
[0142] 5. Cool the mixture obtained in step 4 to below 40°C, and stir until a smooth matrix is formed;
[0143] 6. Weigh and add chlorhexidine, stir and mix until uniform, to ensure that chlorhexidine is evenly dispersed in the matri...
example 2
[0147] Example 2: Formulation and production - including thickeners and preservatives
[0148] Table 2 describes preferred formulations containing thickeners and preservatives.
[0149] Table 2 Formulations Containing Thickeners and Preservatives
[0150]
[0151] Below is the manufacturing process of producing above-mentioned preparation:
[0152] 1. Take heavy liquid paraffin barium sulfate, aluminum stearate, oxygen phase silicon dioxide 200 (Aerosol200), methylparaben and propylparaben;
[0153] 2. Heat the liquid paraffin to 80°C. Keeping at this temperature, add barium sulfate, aluminum stearate, oxysilica 200, methyl p-hydroxybenzoate and propyl p-hydroxybenzoate to the liquid paraffin and stir; continue stirring until a smooth suspension is formed;
[0154] 3. Heat the mixture obtained in step 2 until the temperature rises to 150°C;
[0155] 4. Keep this temperature in a container with a lid for 2 hours without stirring;
[0156] 5. Cool the mixture obtained in...
example 3
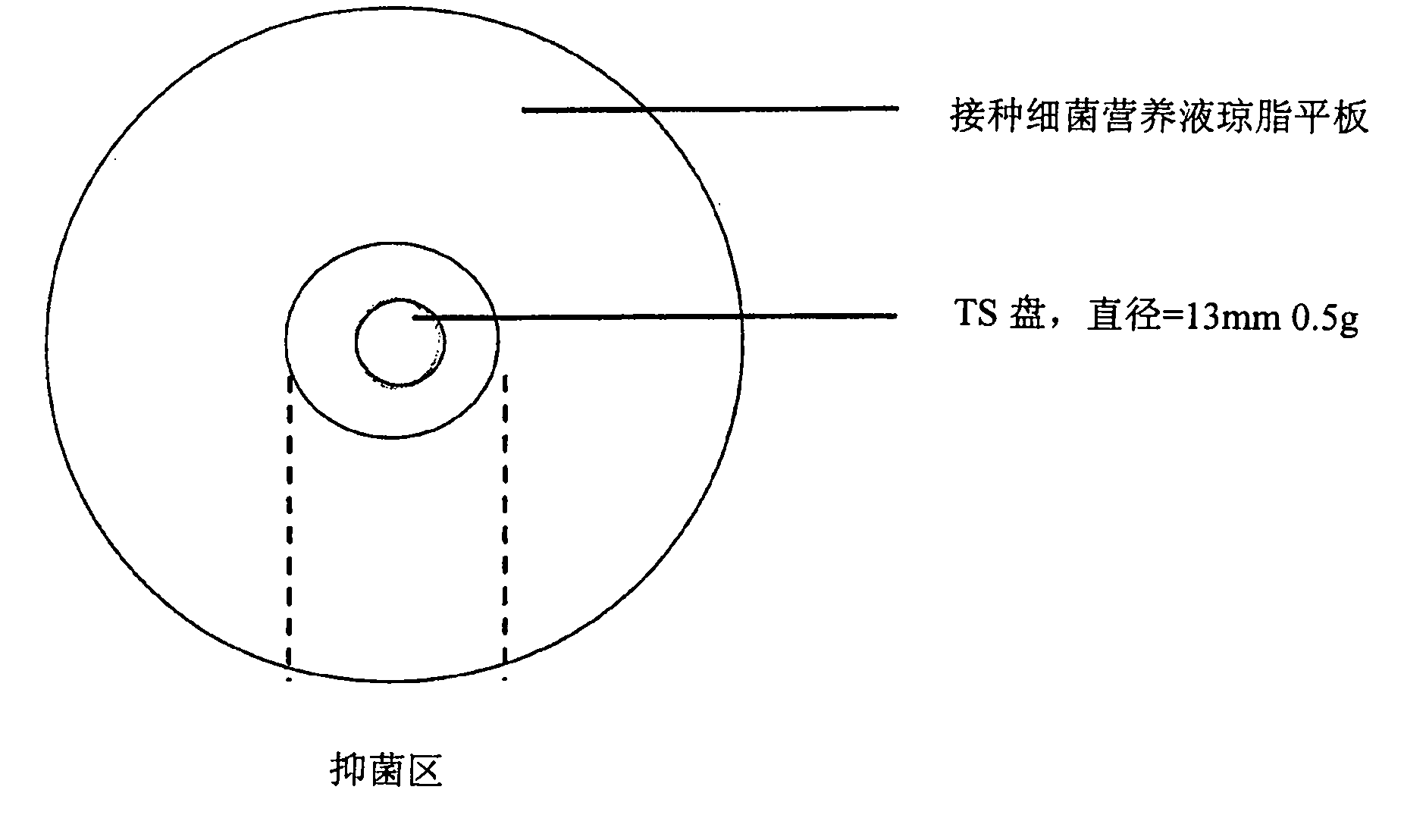
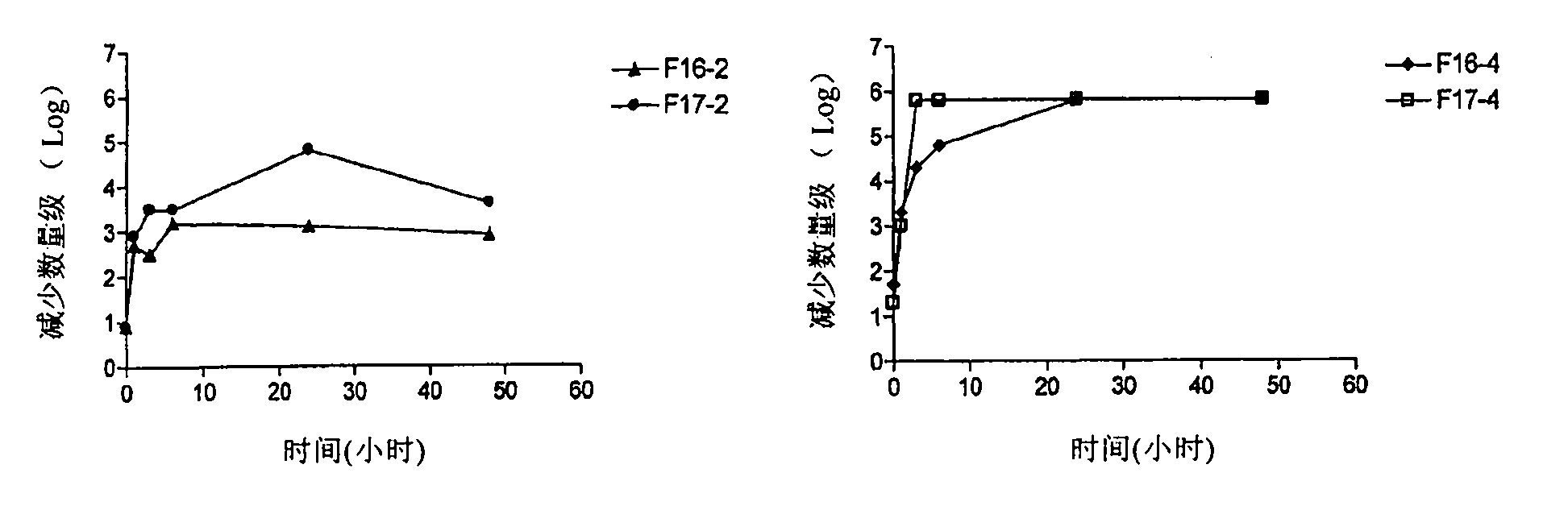
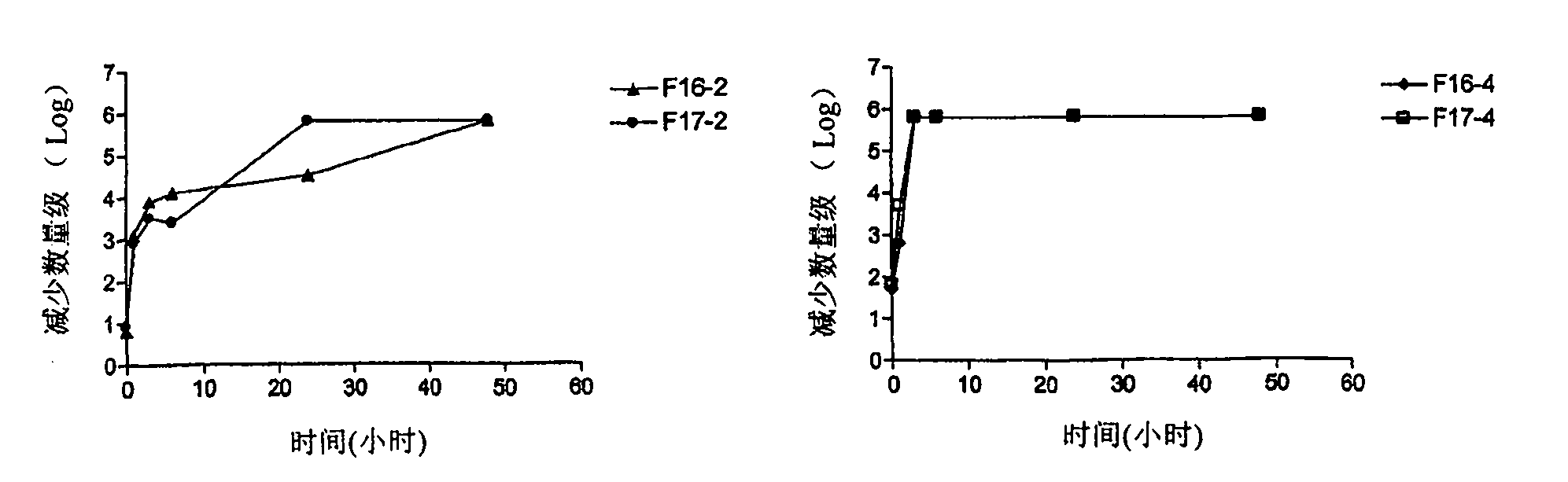
[0161] Example 3: Bacterial Challenge Test Results for Barium Sealants
[0162] The purpose of this experiment was to predict the optimal concentration of a biocide in a formulation that would form a chemical barrier against bacteria proliferating through the teat ducts.
[0163] Another objective was to conduct a detailed study of the factors affecting the release rate of the fungicide, including drug concentration, chemical form (salt or base) and formulation.
[0164] Chlorhexidine at higher concentrations (greater than 100 μg / ml) is bactericidal against most bacteria. Chlorhexidine at lower concentrations (1-100 μg / ml) is used as a bacteriostatic agent (European Medicines Agency).
[0165] Ideally, the formulation should contain chlorhexidine in an amount such that it has a bactericidal concentration after application to the teat ducts and / or the lower part of the teat cisterns. Excessive concentrations should be avoided as high concentrations can cause tissue inflammati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com