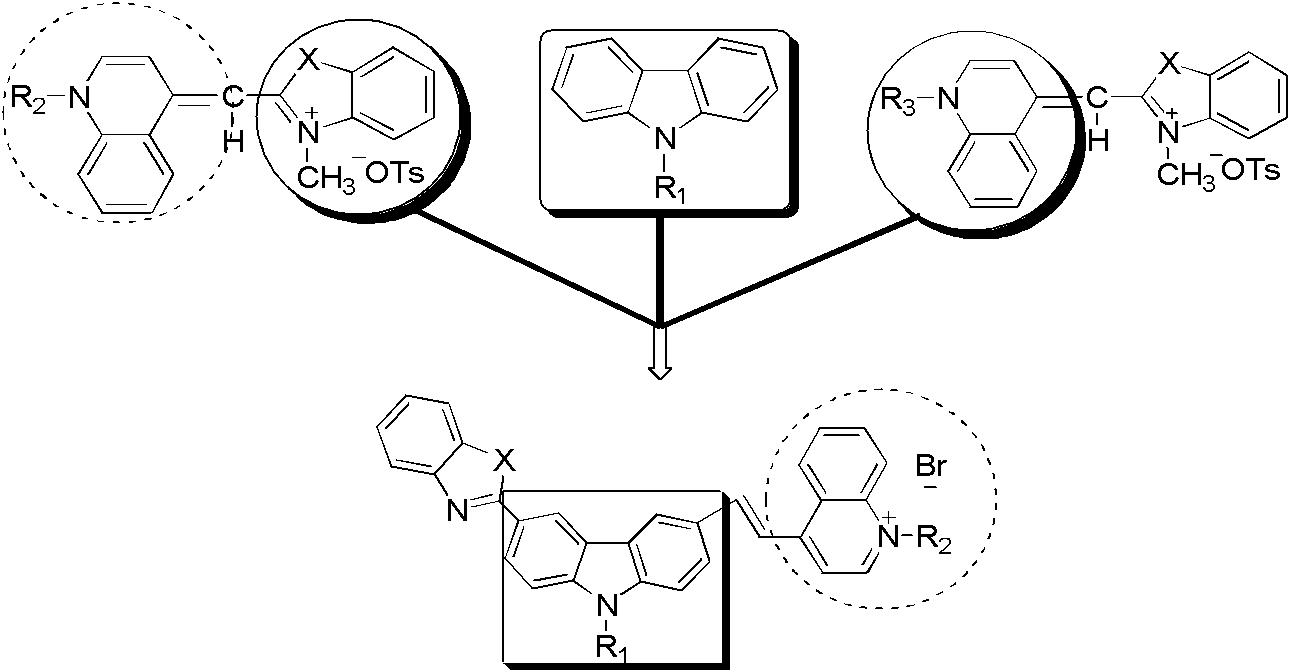
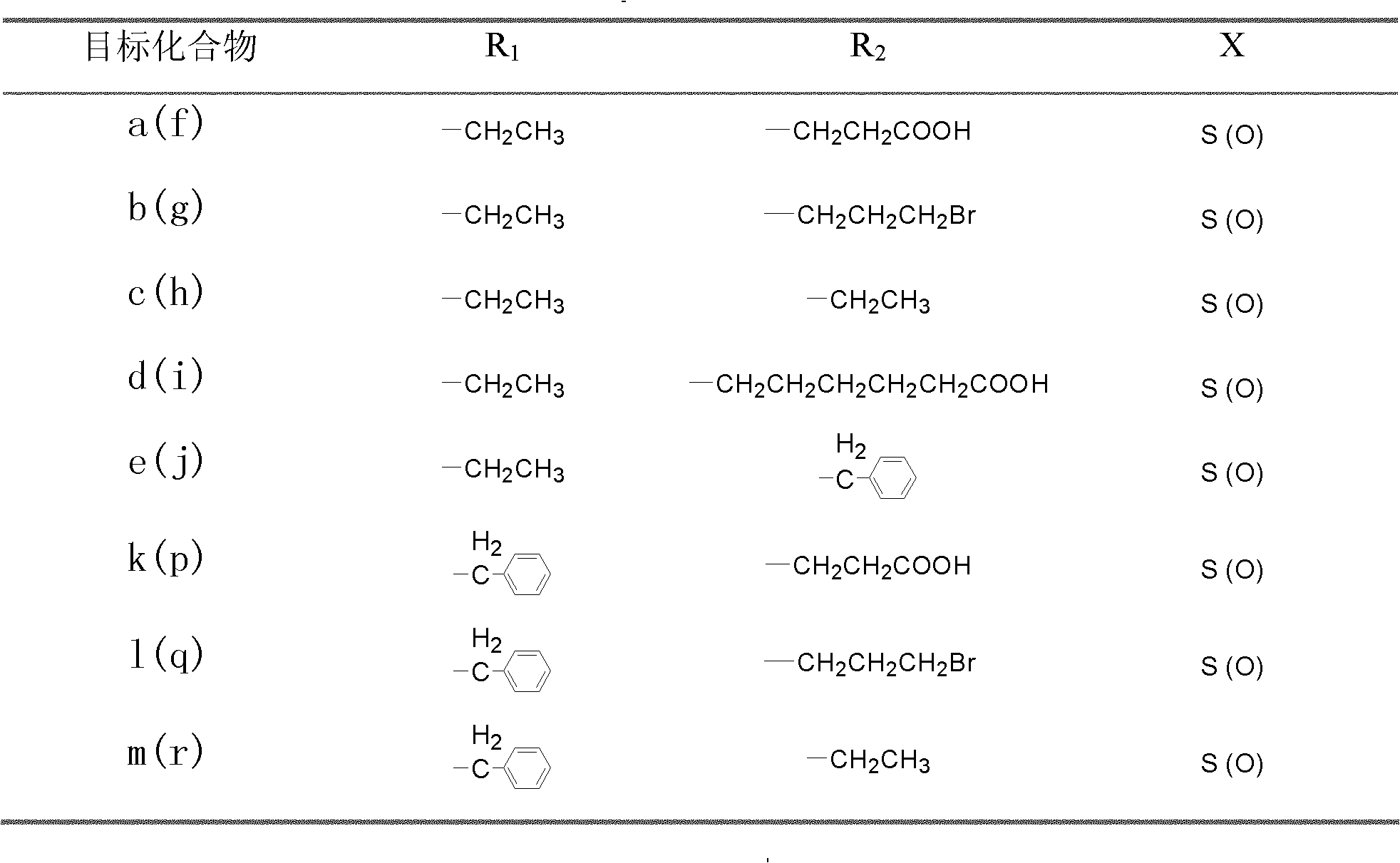Novel carbazole-bridge-based fluorescent cyanine dye probe and preparation method thereof
A carbazole bridge, fluorescent cyanine technology, applied in the field of fluorescent dyes, can solve the problems of small Stocks shift, small maximum fluorescence emission wavelength, weak fluorescence intensity, etc., and achieves increased Stocks shift, large molar extinction coefficient, and increased fluorescence intensity. big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
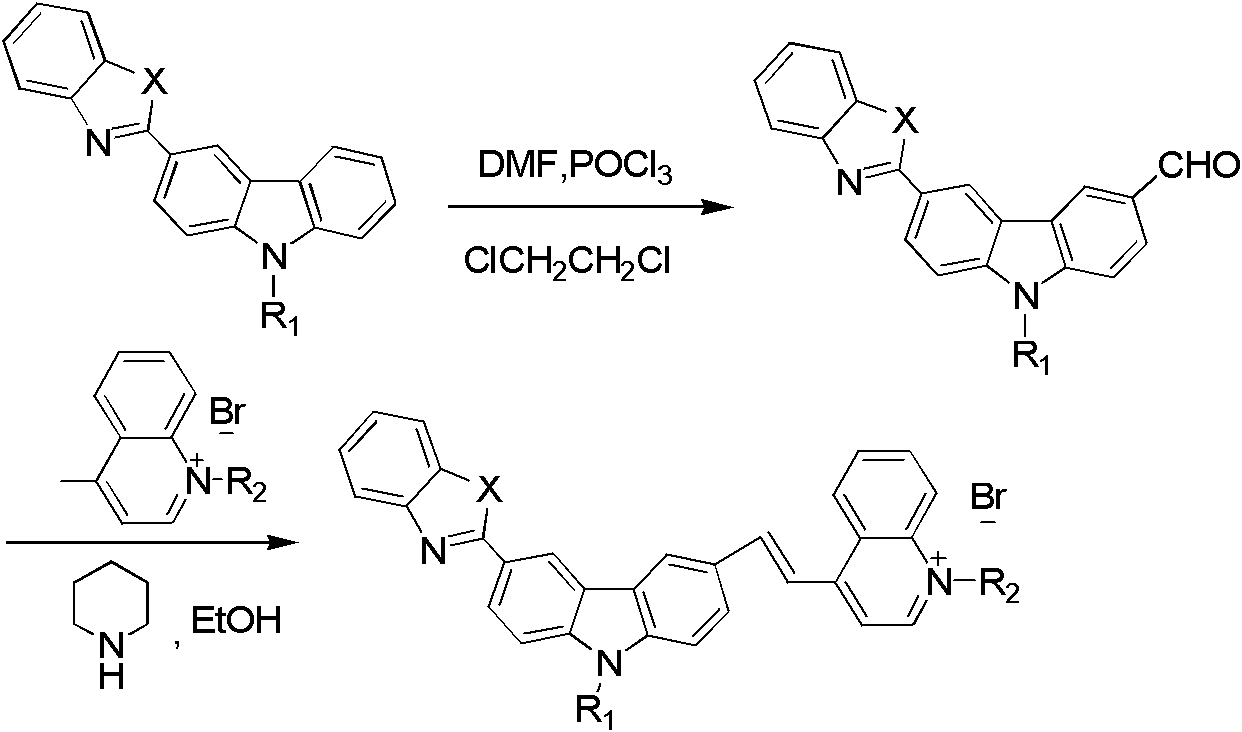
[0024] The preparation method of the carbazole bridging fluorescent cyanine dye probe of the present invention comprises the following steps:
[0025] 1) 3-benzothiazolyl-6-formyl-N-ethylcarbazole, 3-benzoxazolyl-6-formyl-N-ethylcarbazole, 3-benzoxazolyl-6 Preparation of -formyl-N-benzylcarbazole, 3-benzothiazolyl-6-formyl-N-benzylcarbazole
[0026] Add N,N-dimethylformamide (DMF) and phosphorus oxychloride at a ratio of 1:1 to four 100mL round-bottomed flasks respectively, after dropping, stir at room temperature until the reaction system turns reddish. , the amount of substance added dropwise is 3-benzothiazolyl-N-ethylcarbazole, 3-benzoxazolyl-N-ethylcarbazole, 3-benzothiazolyl-N-ethylcarbazole, 3-benzoxazolyl- N-benzylcarbazole, 3-benzothiazolyl-N-benzylcarbazole in 1,2-dichloroethane solution, the total volume is 40-70mL, reflux and stir at the temperature of 79-85°C for 6h -12h, cooled to room temperature, poured into ice water, extracted with dichloromethane, separate...
Embodiment 1
[0035] The preparation of embodiment 1N-ethylcarbazole bridging thiazole orange dye probe comprises the following steps:
[0036] 1.3-Benzothiazolyl-6-formyl-N-ethylcarbazole
[0037] Add 7.7mL (100mmol) of DMF to a 100mL round-bottomed flask, dropwise add 9.5mL (15.2g, 100mmol) of phosphorus oxychloride in an ice-water bath, dropwise, stir at room temperature until the reaction system is reddish, slowly add 3- A solution of 2 g (6 mmol) of benzothiazolyl-N-ethylcarbazole in 30 mL of 1,2-dichloroethane was added and reacted under reflux at 83° C. for 6 h. After cooling to room temperature, it was poured into ice water, extracted with dichloromethane, and separated by column chromatography to obtain 3-benzothiazolyl-6-formyl-N-ethylcarbazole.
[0038] 2. Preparation of N-ethylcarbazole bridging thiazole orange dye probe a
[0039] Dissolve 0.1g (0.28mmol) of 3-benzothiazolyl-6-formyl-N-ethylcarbazole in 30mL of ethanol, then dissolve 0.13g (0.42mmol) of carboxyquinoline salt ...
Embodiment 2
[0041] The preparation of embodiment 2N-benzylcarbazole bridge thiazole orange dye probe comprises the following steps:
[0042] 1. Preparation of 3-benzothiazolyl-6-formyl-N-benzylcarbazole
[0043] Add DMF 7.7mL (100mmol) to the flask, add phosphorus oxychloride 9.5mL (15.2g, 100mmol) dropwise under ice-water bath, after dropping, stir at room temperature until the solution is reddish, slowly add 3-benzothiazolyl - A solution of 2.34g (6mmol) of N-benzylcarbazole in 30mL of 1,2-dichloroethane, after dropping, reflux at 83°C for 16h. After cooling to room temperature, it was poured into ice water, extracted with dichloromethane, and separated by column chromatography to obtain 3-benzothiazolyl-6-formyl-N-benzylcarbazole.
[0044] 2. Preparation of N-benzylcarbazole bridging thiazole orange dye probe k
[0045] Dissolve 0.1g (0.28mmol) of 3-benzothiazolyl-6-formyl-N-benzylcarbazole in 30mL of ethanol, then dissolve 0.13g (0.42mmol) of carboxyquinoline salt in 20mL of ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com