Cathode material of lithium ion battery and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, which is applied to battery electrodes, circuits, electrical components, etc., can solve the problems of large solvent consumption, difficult to strictly control the ratio, poor repeatability of precursors, etc., and achieves a simple and easy preparation method. The effect of large-scale production and excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of lithium nickel manganese cobalt oxide
[0027] LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 and C 6 h 8 o 7 (LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 and C 6 h 8 o 7The molar ratio is 1.07:0.33:0.33:0.33:1.2) was added into water, mixed evenly, and the pH of the system was adjusted to 7; the system was heated to 50°C for 12h to obtain a transparent sol; the sol was dried at 80°C for 90h, Obtain the precursor powder; pre-sinter the precursor powder at 300°C for 8h to obtain the intermediate product powder, press the intermediate product powder into tablets and sinter at 900°C for 12h to obtain LiNi x mn y co 1-x-y o 2 Nanoparticles, where x is 0.33 and y is 0.33.
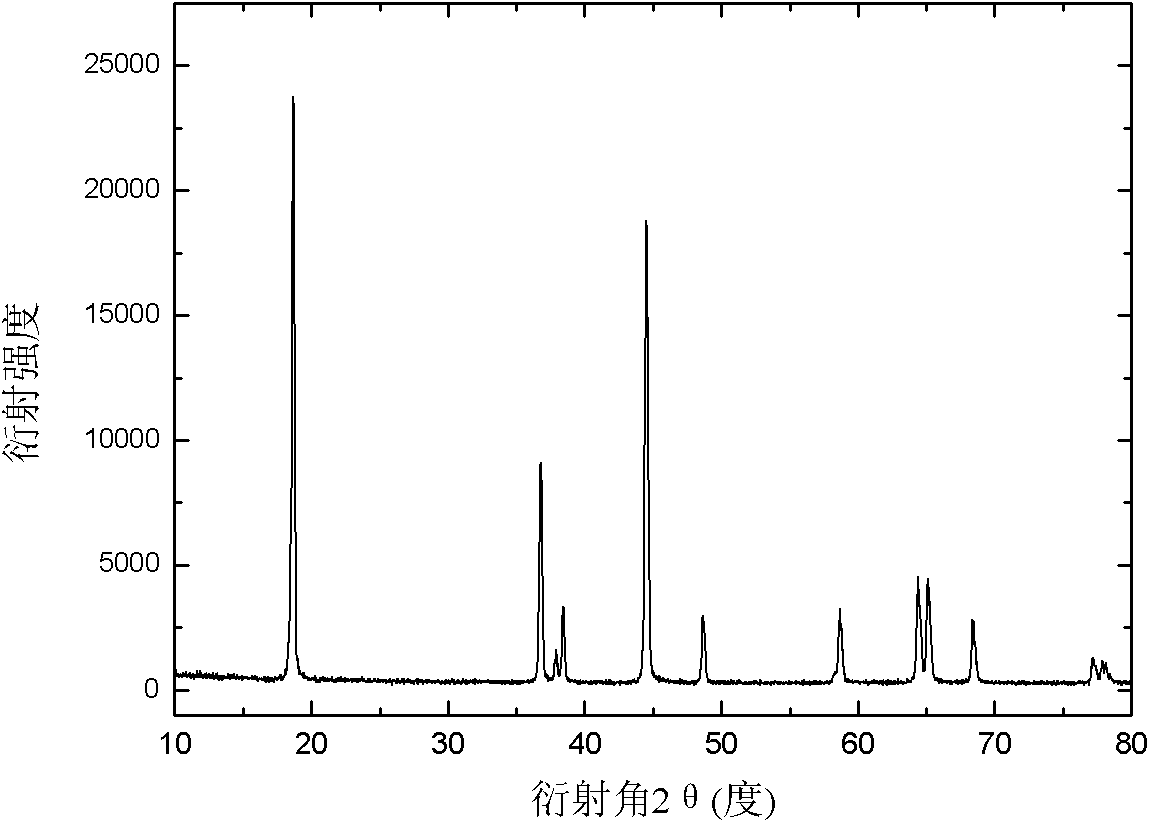
[0028] Powder X-ray diffractometer (Rigaku DmaxrB, CuK α ray) to analyze the product, the results are as follows figure 1 shown; from figure 1 It can be seen that there is no impurity peak in the spectrogram, indicating that the product is ...
Embodiment 2
[0031] Embodiment 2, the preparation of lithium nickel manganese cobalt oxygen
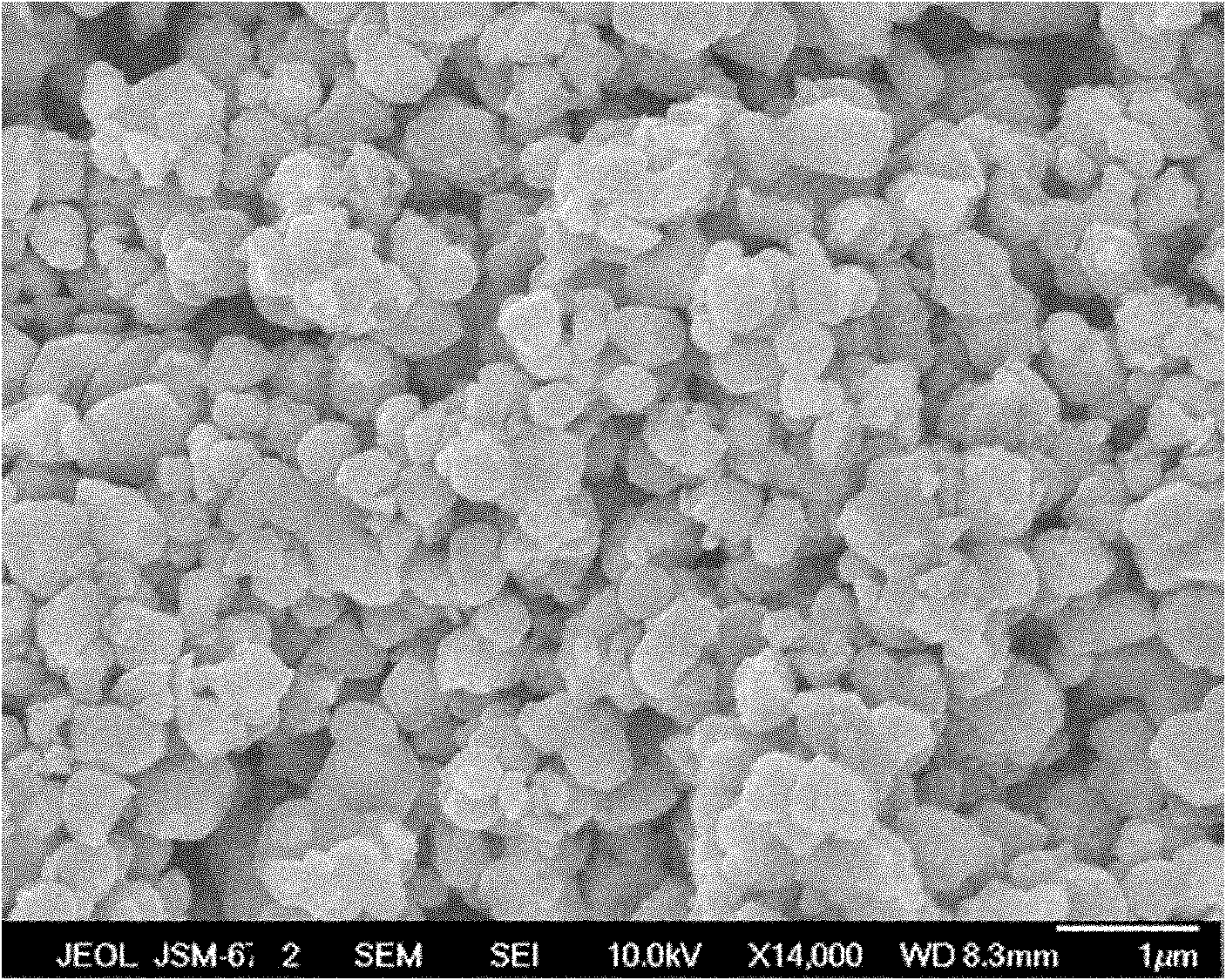
[0032] LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 and C 6 h 8 o 7 (LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 and C 6 h 8 o 7 The molar ratio is 1.05:0.4:0.4:0.2:1) into water, mix well, adjust the pH of the system to 6; heat to 50°C for 12h to obtain a transparent sol; dry the sol at 80°C for 90h to obtain Precursor powder; pre-sinter the precursor powder at 400°C for 6h to obtain intermediate product powder; press the intermediate product powder into tablets and sinter at 900°C for 10h to obtain LiNi x mn y co 1-x-y o 2 The nanoparticles have a particle diameter of 20nm-700nm, wherein x is 0.4 and y is 0.4.
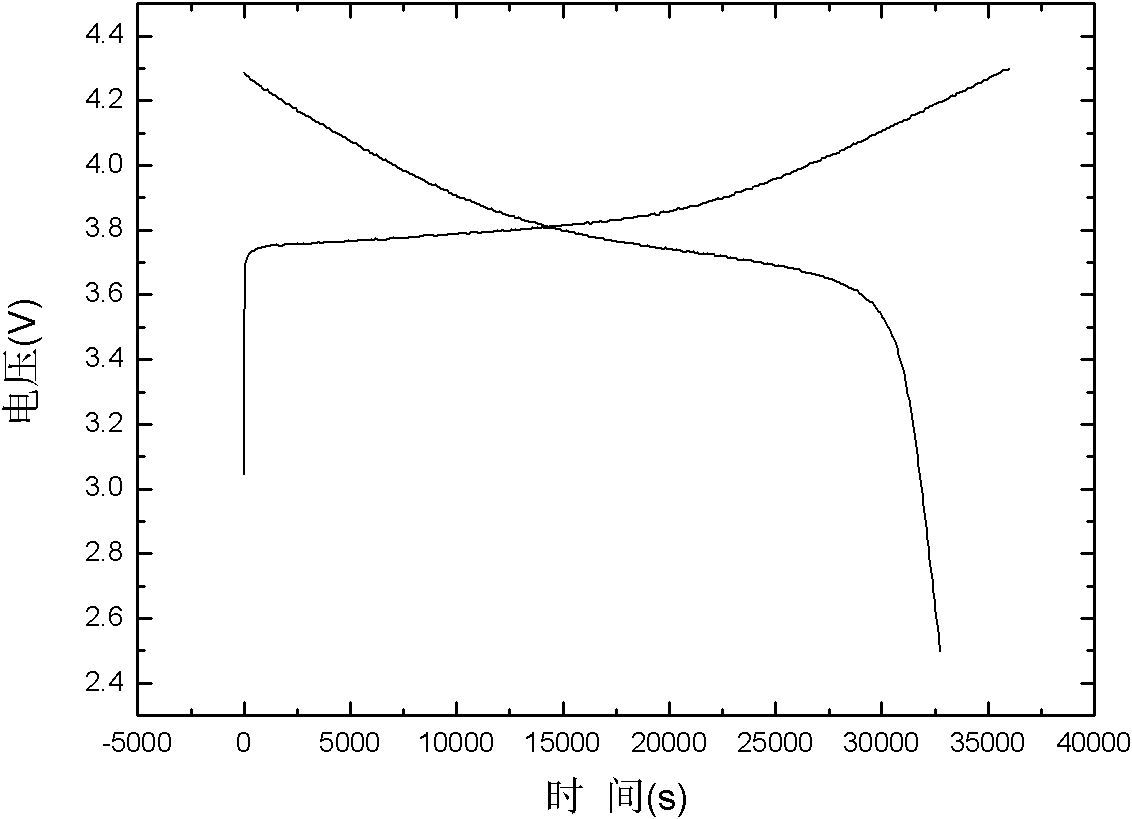
[0033] The specific discharge capacity of lithium nickel manganese cobalt oxygen prepared by this embodiment is 159mAh / g.
Embodiment 3
[0034] Embodiment 3, the preparation of lithium nickel manganese cobalt oxide
[0035] LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 and C 6 h 8 o 7 (LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 and C 6 h 8 o 7 The molar ratio is 1.03:0.5:0.3:0.2:0.8) into water, mix uniformly, adjust the pH of the system to 5; heat to 60°C and react for 10h to obtain a transparent sol; dry the sol at 80°C for 90h to obtain Precursor powder; pre-sintering the precursor powder at 500°C for 5 hours to obtain intermediate product powder; pressing the intermediate product powder into tablets and sintering at 900°C for 18 hours to obtain LiNi x mn y co 1-x-y o 2 The nanoparticles have a particle diameter of 10nm-500nm, wherein x is 0.5 and y is 0.3.
[0036] The specific discharge capacity of lithium nickel manganese cobalt oxygen prepared by this example is 161mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com