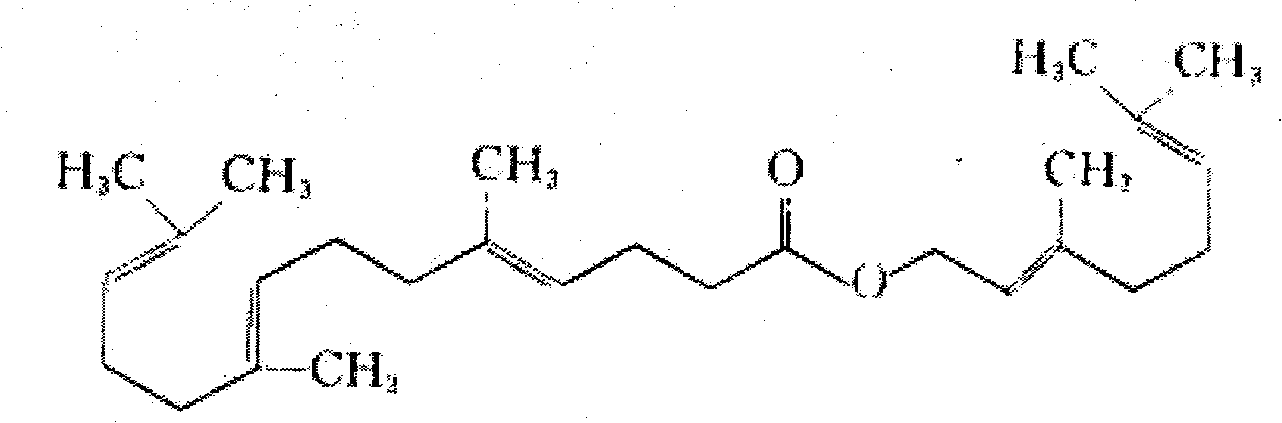
Process for synthesizing gefarnate compound
A synthetic process, gefar ester technology, applied in the field of medicine, can solve the problems of large environmental pollution, volatile, unfavorable industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0022] Specific example: add 5000g triethyl orthoacetate, 1375g tertiary nerolidol, 55g isobutyric acid into the reaction flask, stir to dissolve, heat under stirring, slowly distill the 50-90°C fraction at 120-160°C, and distill for 48 hours , down to room temperature, rectification under reduced pressure, collect 140-145 ℃ fraction, obtain yellow oily matter intermediate-1077g, yield 60%; Add ethanol 4315ml, potassium hydroxide (content 82%) 295g in reaction bottle, stir Dissolve, then add 1050g of intermediate 1, heat to 55°C, keep warm for 8 hours, evaporate ethanol to dryness under reduced pressure, add 8630ml of water, stir to dissolve, extract three times with ethyl acetate 2875ml*3, use 3mol / L hydrochloric acid for the water phase Adjust the pH value of 1440ml to be equal to 3, extract twice with ethyl acetate 2875ml*2, wash with 5000ml*3 water three times, dry, filter, and evaporate the solvent under reduced pressure to obtain 882.5g of yellow oily intermediate 2 with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com