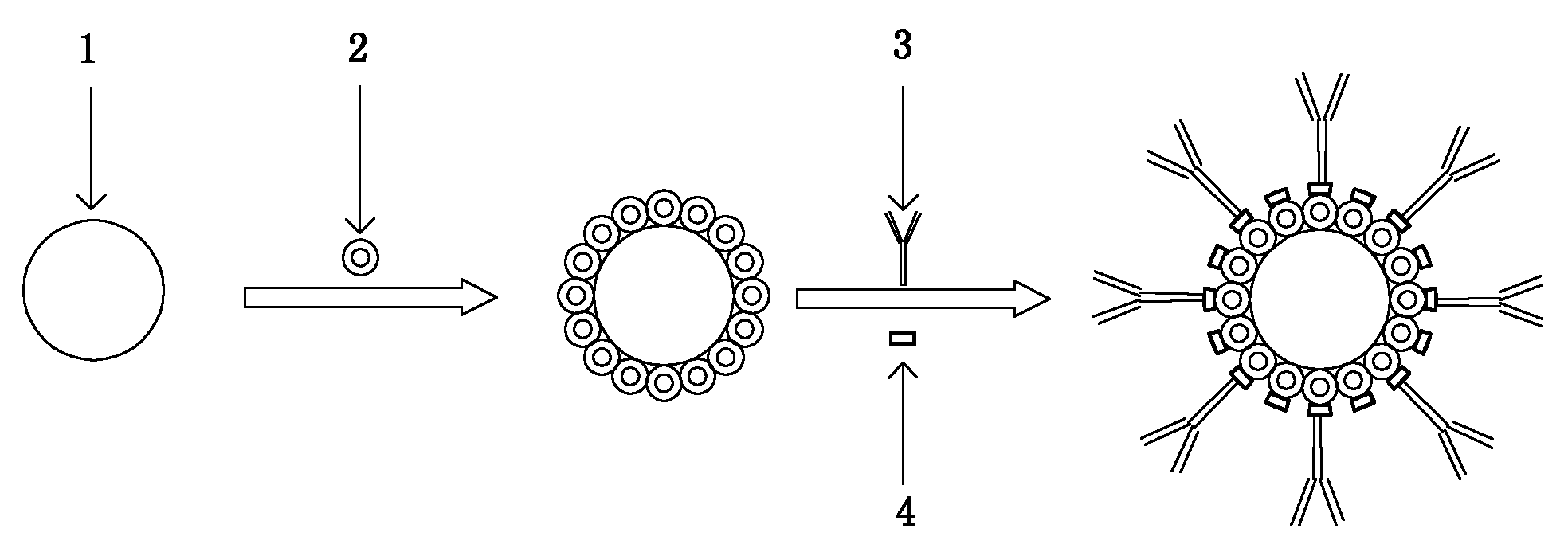
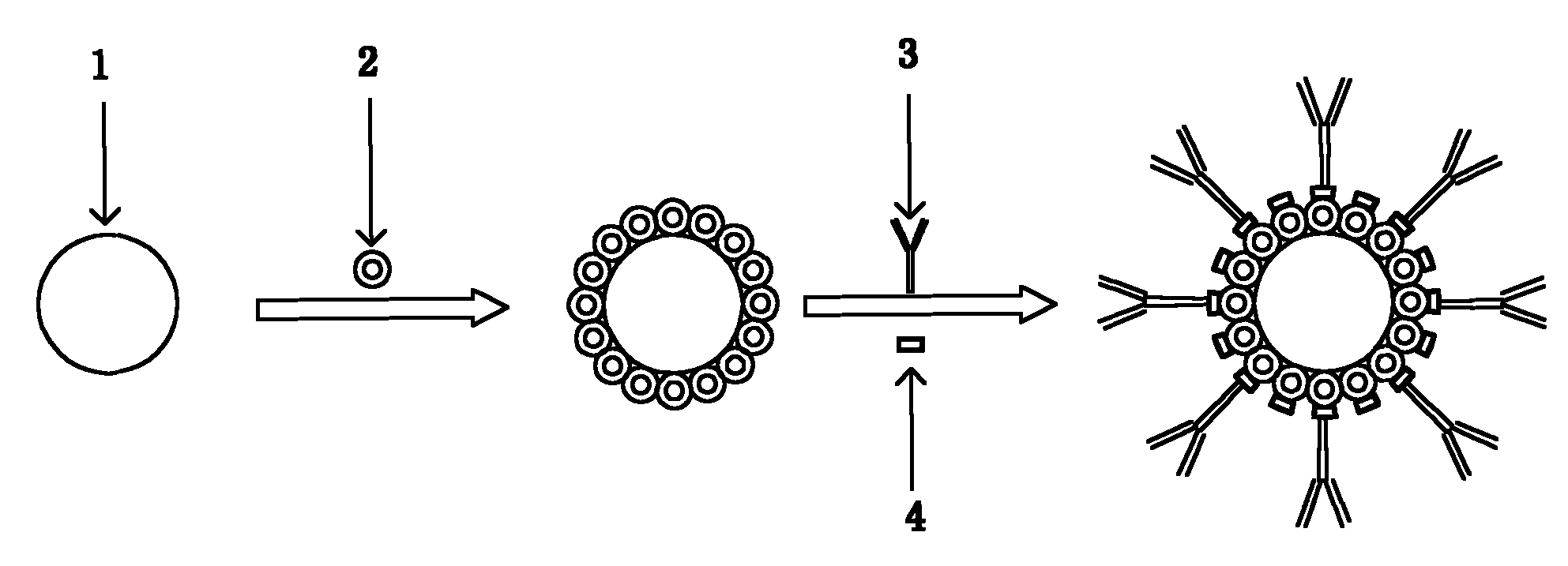
Method for preparing enzyme labelling antibody
An enzyme-labeled antibody and labeling technology, which is applied in the field of immunoassay to achieve the effect of increasing the connection amount, improving the signal amplification ability, and increasing the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 2.5mL of 1% sodium citrate aqueous solution and add it to the boiling 100mL of 0.01% chloroauric acid solution, and at the same time perform magnetic stirring to make it evenly mixed. After reacting for a period of time, the solution turns from yellow to blue, and finally turns into wine red. Stop heating and keep cooling under the condition of magnetic stirring to obtain colloidal gold with a diameter of about 18nm.
[0024] Take a certain volume of colloidal gold solution, centrifuge at 15000g and 4°C for 15min, suck off the supernatant, and redissolve with half the original volume of deionized water. with 0.2M K 2 CO 3Adjust the pH value of the concentrated colloidal gold to about 8.2-8.5, and then add a certain concentration of horseradish peroxidase (HRP, isoelectric point PI = 7.2-8.0), so that the final concentration of HRP is 50 μg / mL. Rotate and mix at room temperature or 4°C for 1-2 hours, then centrifuge the reaction solution at 15,000g and 4°C, absorb...
Embodiment 2
[0027] Get 1.5mL of 1% sodium citrate aqueous solution and add to the boiling 100mL containing 0.01% chloroauric acid solution, and colloidal gold with a diameter of about 25nm can be obtained according to the steps in Example 1.
[0028] Concentrate the colloidal gold with 0.1M hydrochloric acid to adjust the pH value to about 5, then add a certain concentration of alkaline phosphatase (AP, isoelectric point PI = 4.5), so that the final concentration of AP is 50 μg / mL. . Except that N,N'-disuccinimide carbonate is used as the bifunctional reagent (the final concentration of N,N'-disuccinimide carbonate in the solution is 0.2% to 1%), the rest of the steps are the same as in Example 1.
Embodiment 3
[0030] Add 1.2mL of 1% sodium citrate aqueous solution into 100mL of boiled 0.01% chloroauric acid solution, and follow the steps in Example 1 to obtain colloidal gold with a diameter of about 40nm.
[0031] Concentrate the colloidal gold with 0.1M hydrochloric acid to adjust the pH value to about 5 with the steps in Example 1, and then add a certain concentration of galactosidase (β-Gal, isoelectric point PI =4.6) to make the final concentration of β-Gal 100μg / mL. The remaining steps were the same as in Example 1, except that the final concentration of the added antibody was changed to 50 μg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com